The spatiospectral characterization of brain networks: fusing concurrent EEG spectra and fMRI maps
- PMID: 23266744
- PMCID: PMC3568990
- DOI: 10.1016/j.neuroimage.2012.12.024
The spatiospectral characterization of brain networks: fusing concurrent EEG spectra and fMRI maps
Abstract
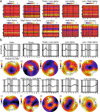
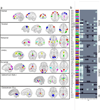
Different imaging modalities capture different aspects of brain activity. Functional magnetic resonance imaging (fMRI) reveals intrinsic networks whose BOLD signals have periods from 100 s (0.01 Hz) to about 10s (0.1 Hz). Electroencephalographic (EEG) recordings, in contrast, commonly reflect cortical electrical fluctuations with periods up to 20 ms (50 Hz) or above. We examined the correspondence between intrinsic fMRI and EEG network activity at rest in order to characterize brain networks both spatially (with fMRI) and spectrally (with EEG). Brain networks were separately identified within the concurrently recorded fMRI and EEG at the aggregate group level with group independent component analysis and the association between spatial fMRI and frequency by spatial EEG sources was examined by deconvolving their component time courses. The two modalities are considered linked if the estimated impulse response function (IRF) is significantly non-zero at biologically plausible delays. We found that negative associations were primarily present within two of five alpha components, which highlights the importance of considering multiple alpha sources in EEG-fMRI. Positive associations were primarily present within the lower (e.g. delta and theta) and higher (e.g. upper beta and lower gamma) spectral regions, sometimes within the same fMRI components. Collectively, the results demonstrate a promising approach to characterize brain networks spatially and spectrally, and reveal that positive and negative associations appear within partially distinct regions of the EEG spectrum.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures



References
-
- Aguirre GK, Zarahn E, D’Esposito M. The variability of human, BOLD hemodynamic responses. Neuroimage. 1998;8:360–369. - PubMed
-
- Beckmann CF, Smith SM. Tensorial extensions of independent component analysis for multisubject FMRI analysis. Neuroimage. 2005;25(1):294–311. - PubMed
-
- Bell AJ, Sejnowski TJ. An information-maximization approach to blind separation and blind deconvolution. Neural computation. 1995;7(6):1129–1159. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

