Nuclease digestion and mass spectrometric characterization of oligodeoxyribonucleotides containing 1,2-GpG, 1,2-ApG, and 1,3-GpXpG cisplatin intrastrand cross-links
- PMID: 23266768
- PMCID: PMC3625460
- DOI: 10.1016/j.cca.2012.12.010
Nuclease digestion and mass spectrometric characterization of oligodeoxyribonucleotides containing 1,2-GpG, 1,2-ApG, and 1,3-GpXpG cisplatin intrastrand cross-links
Abstract
Background: The primary mode of action for cis-diamminedichloroplatinum (II), referred to as cisplatin, toward the treatment of solid malignancies is through formation of cross-links with DNA at purine sites, especially guanines.
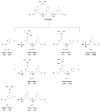
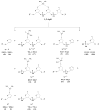
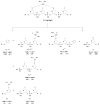
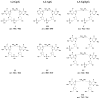
Methods: We prepared oligodeoxyribonucleotides (ODNs) containing a 1,2-GpG, 1,2-ApG, or 1,3-GpXpG cisplatin intrastrand cross-link and the corresponding ODNs modified with (15)N2-labeled cisplatin, and characterized these ODNs with electrospray ionization mass spectrometry (ESI-MS) and tandem MS (MS/MS). We also employed LC-MS/MS to characterize the digestion products of these ODNs after treatment with a cocktail of 4 enzymes (nuclease P1, phosphodiesterases I and II, and alkaline phosphatase).
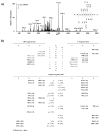
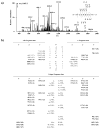
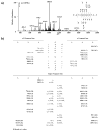
Results: 1,2-GpG was released from the ODNs as a dinucleoside monophosphate or a dinucleotide. Analyses of the digestion products of ODNs containing a 1,2-GpG cross-link on the 5' or 3' terminus revealed that the dinucleotide carries a terminal 5' phosphate. On the other hand, digestion of the 1,3-GpXpG intrastrand cross-link yielded 3 dinucleoside products with 0, 1, or 2 phosphate groups.
Conclusion: The availability of the ODNs carrying the stable isotope-labeled lesions, MS/MS analyses of the cisplatin-modified ODNs, and the characterization of the enzymatic digestion products of these ODNs set the stage for the future LC-MS/MS quantification of the 1,2-GpG, 1,2-ApG, and 1,3-GpXpG lesions in cellular DNA.
Copyright © 2012 Elsevier B.V. All rights reserved.
Figures


Similar articles
-
Mass spectrometry of cis-diamminedichloroplatinum(II) adducts with the dinucleosidemonophosphates d(ApG), d(GpG) and d(TpC) in an ion trap.J Mass Spectrom. 2002 Jul;37(7):731-47. doi: 10.1002/jms.333. J Mass Spectrom. 2002. PMID: 12125006
-
RNA polymerases react differently at d(ApG) and d(GpG) adducts in DNA modified by cis-diamminedichloroplatinum(II).Biochemistry. 1992 Feb 25;31(7):1904-8. doi: 10.1021/bi00122a002. Biochemistry. 1992. PMID: 1536834
-
A 1,2-d(GpG) cisplatin intrastrand cross-link influences the rotational and translational setting of DNA in nucleosomes.J Am Chem Soc. 2008 Mar 5;130(9):2851-61. doi: 10.1021/ja710220x. Epub 2008 Feb 13. J Am Chem Soc. 2008. PMID: 18269283 Free PMC article.
-
A rare example of three abundant conformers in one retro model of the cisplatin-DNA d(GpG) intrastrand cross link. Unambiguous evidence that guanine O6 to carrier amine ligand hydrogen bonding is not important. possible effect of the Lippard base pair step adjacent to the lesion on carrier ligand hydrogen bonding in DNA adducts.J Am Chem Soc. 2001 Sep 26;123(38):9345-55. doi: 10.1021/ja010483m. J Am Chem Soc. 2001. PMID: 11562217
-
Trans-diammineplatinum(II): what makes it different from cis-DDP? Coordination chemistry of a neglected relative of cisplatin and its interaction with nucleic acids.Met Ions Biol Syst. 1996;33:105-41. Met Ions Biol Syst. 1996. PMID: 8742842 Review.
Cited by
-
Multi-adductomics: Advancing mass spectrometry techniques for comprehensive exposome characterization.Trends Analyt Chem. 2024 Nov;180:117900. doi: 10.1016/j.trac.2024.117900. Epub 2024 Aug 5. Trends Analyt Chem. 2024. PMID: 39246549
-
Cisplatin intrastrand adducts sensitize DNA to base damage by hydrated electrons.J Phys Chem B. 2014 May 8;118(18):4803-8. doi: 10.1021/jp5014913. Epub 2014 Apr 29. J Phys Chem B. 2014. PMID: 24779712 Free PMC article.
References
-
- Jamieson ER, Lippard SJ. Structure, recognition, and processing of cisplatin-DNA adducts. Chem Rev. 1999;99:2467–2498. - PubMed
-
- Siddik ZH. Cisplatin: Mode of cytotoxic action and molecular basis of resistance. Oncogene. 2003;22:7265–7279. - PubMed
-
- Jung Y, Lippard SJ. Direct cellular responses to platinum-induced DNA damage. Chem Rev. 2007;107:1387–1407. - PubMed
-
- Kozelka J, Legendre F, Reeder F, Chottard J-C. Kinetic aspects of interactions between DNA and platinum complexes. Coord Chem Rev. 1999;190–192:61–82.
-
- Robertazzi A, Platts JA. A QM/MM study of cisplatin–DNA oligonucleotides: from simple models to realistic systems. Chem Eur J. 2006;12:5747–5756. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

