'Roid rage in rats? Testosterone effects on aggressive motivation, impulsivity and tyrosine hydroxylase
- PMID: 23266798
- PMCID: PMC3615053
- DOI: 10.1016/j.physbeh.2012.12.005
'Roid rage in rats? Testosterone effects on aggressive motivation, impulsivity and tyrosine hydroxylase
Abstract
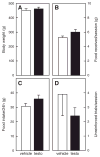
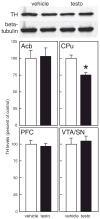
In humans and animals, anabolic-androgenic steroids (AAS) increase aggression, but the underlying behavioral mechanisms are unclear. AAS may increase the motivation to fight. Alternatively, AAS may increase impulsive behavior, consistent with the popular image of 'roid rage. To test this, adolescent male rats were treated chronically with testosterone (7.5mg/kg) or vehicle and tested for aggressive motivation and impulsivity. Rats were trained to respond on a nose-poke on a 10 min fixed-interval schedule for the opportunity to fight in their home cage with an unfamiliar rat. Although testosterone increased aggression (6.3±1.3 fights/5 min vs 2.4±0.8 for controls, p<0.05), there was no difference in operant responding (28.4±1.6 nose-pokes/10 min for testosterone, 32.4±7.0 for vehicle). This suggests that testosterone does not enhance motivation for aggression. To test for impulsivity, rats were trained to respond for food in a delay-discounting procedure. In an operant chamber, one lever delivered one food pellet immediately, the other lever gave 4 pellets after a delay (0, 15, 30 or 45 s). In testosterone- and vehicle-treated rats, body weights and food intake did not differ. However, testosterone-treated rats chose the larger, delayed reward more often (4.5±0.7 times in 10 trials with 45 s delay) than vehicle controls (2.5±0.5 times, p<0.05), consistent with a reduction in impulsive choice. Thus, although chronic high-dose testosterone enhances aggression, this does not include an increase in impulsive behavior or motivation to fight. This is further supported by measurement of tyrosine hydroxylase (TH) by Western immunoblot analysis in brain regions important for motivation (nucleus accumbens, Acb) and executive function (medial prefrontal cortex, PFC). There were no differences in TH between testosterone- and vehicle-treated rats in Acb or PFC. However, testosterone significantly reduced TH (to 76.9±3.1% of controls, p<0.05) in the caudate-putamen, a brain area important for behavioral inhibition, motor control and habit learning.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures


References
-
- Brower KJ, Blow FC, Young JP, Hill EM. Symptoms and correlates of anabolic-androgenic steroid dependence. Br J Addict. 1991;86:759–68. - PubMed
-
- Conacher GN, Workman DG. Violent crime possibly associated with anabolic steroid use. Am J Psychiatry. 1989;146:679. - PubMed
-
- Pope HG, Jr, Katz DL. Homicide and near-homicide by anabolic steroid users. J Clin Psychiatry. 1990;51:28–31. - PubMed
-
- Pope HG, Jr, Kouri EM, Powell KF, Campbell C, Katz DL. Anabolic-androgenic steroid use among 133 prisoners. Compr Psychiatry. 1996;37:322–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

