Neutrophil extracellular trap-associated protein activation of the NLRP3 inflammasome is enhanced in lupus macrophages
- PMID: 23267025
- PMCID: PMC3552129
- DOI: 10.4049/jimmunol.1202388
Neutrophil extracellular trap-associated protein activation of the NLRP3 inflammasome is enhanced in lupus macrophages
Abstract
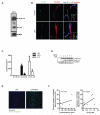
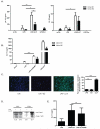
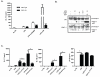
Neutrophil extracellular traps (NETs) represent an important defense mechanism against microorganisms. Clearance of NETs is impaired in a subset of patients with systemic lupus erythematosus, and NETosis is increased in neutrophils and, particularly, in low-density granulocytes derived from lupus patients. NETs are toxic to the endothelium, expose immunostimulatory molecules, activate plasmacytoid dendritic cells, and may participate in organ damage through incompletely characterized pathways. To better understand the role of NETs in fostering dysregulated inflammation, we examined inflammasome activation in response to NETs or to LL-37, an antibacterial protein externalized on NETs. Both NETs and LL-37 activate caspase-1, the central enzyme of the inflammasome, in both human and murine macrophages, resulting in release of active IL-1β and IL-18. LL-37 activation of the NLRP3 inflammasome utilizes P2X7 receptor-mediated potassium efflux. NET and LL-37-mediated activation of the inflammasome is enhanced in macrophages derived from lupus patients. In turn, IL-18 is able to stimulate NETosis in human neutrophils. These results suggest that enhanced formation of NETs in lupus patients can lead to increased inflammasome activation in adjacent macrophages. This leads to release of inflammatory cytokines that further stimulate NETosis, resulting in a feed-forward inflammatory loop that could potentially lead to disease flares and/or organ damage.
Figures
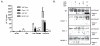


Similar articles
-
TLR agonists stimulate Nlrp3-dependent IL-1β production independently of the purinergic P2X7 receptor in dendritic cells and in vivo.J Immunol. 2013 Jan 1;190(1):334-9. doi: 10.4049/jimmunol.1202737. Epub 2012 Dec 7. J Immunol. 2013. PMID: 23225887 Free PMC article.
-
Bruton's tyrosine kinase (BTK) and matrix metalloproteinase-9 (MMP-9) regulate NLRP3 inflammasome-dependent cytokine and neutrophil extracellular trap responses in primary neutrophils.J Allergy Clin Immunol. 2025 Feb;155(2):569-582. doi: 10.1016/j.jaci.2024.10.035. Epub 2024 Nov 14. J Allergy Clin Immunol. 2025. PMID: 39547282
-
The purinergic 2X7 receptor participates in renal inflammation and injury induced by high-fat diet: possible role of NLRP3 inflammasome activation.J Pathol. 2013 Nov;231(3):342-53. doi: 10.1002/path.4237. Epub 2013 Sep 3. J Pathol. 2013. PMID: 23843215
-
From innate immunity to autoimmunity: Neutrophils in systemic lupus erythematosus pathogenesis.Lupus. 2025 Sep;34(10):989-1002. doi: 10.1177/09612033251352734. Epub 2025 Jun 18. Lupus. 2025. PMID: 40531091 Review.
-
The inflammasome and lupus: another innate immune mechanism contributing to disease pathogenesis?Curr Opin Rheumatol. 2014 Sep;26(5):475-81. doi: 10.1097/BOR.0000000000000088. Curr Opin Rheumatol. 2014. PMID: 24992143 Free PMC article. Review.
Cited by
-
Unknown/enigmatic functions of extracellular ASC.Immunology. 2021 Aug;163(4):377-388. doi: 10.1111/imm.13375. Epub 2021 Jun 22. Immunology. 2021. PMID: 34042182 Free PMC article. Review.
-
Increased neutrophil extracellular trap formation in oligoarticular, polyarticular juvenile idiopathic arthritis and enthesitis-related arthritis: biomarkers for diagnosis and disease activity.Front Immunol. 2024 Aug 9;15:1436193. doi: 10.3389/fimmu.2024.1436193. eCollection 2024. Front Immunol. 2024. PMID: 39185410 Free PMC article.
-
Metabolic Factors that Contribute to Lupus Pathogenesis.Crit Rev Immunol. 2016;36(1):75-98. doi: 10.1615/CritRevImmunol.2016017164. Crit Rev Immunol. 2016. PMID: 27480903 Free PMC article. Review.
-
LL37:DNA complexes provide antimicrobial activity against intracellular bacteria in human macrophages.Immunology. 2016 Aug;148(4):420-32. doi: 10.1111/imm.12620. Immunology. 2016. PMID: 27177697 Free PMC article.
-
Myeloid Cells in Myocardial Ischemic Injury: The Role of the Macrophage Migration Inhibitory Factor.Life (Basel). 2024 Aug 5;14(8):981. doi: 10.3390/life14080981. Life (Basel). 2024. PMID: 39202723 Free PMC article. Review.
References
-
- Brinkmann V, Zychlinsky A. Beneficial suicide: why neutrophils die to make NETs. Nat Rev Microbiol. 2007;5:577–582. - PubMed
-
- Knight JS, Kaplan MJ. Lupus neutrophils: ‘NET’ gain in understanding lupus pathogenesis. Current opinion in rheumatology. 2012;24:441–450. - PubMed
-
- Lande R, Ganguly D, Facchinetti V, Frasca L, Conrad C, Gregorio J, Meller S, Chamilos G, Sebasigari R, Riccieri V, Bassett R, Amuro H, Fukuhara S, Ito T, Liu YJ, Gilliet M. Neutrophils Activate Plasmacytoid Dendritic Cells by Releasing Self-DNA-Peptide Complexes in Systemic Lupus Erythematosus. Sci Transl Med. 2011;3:73ra19. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

