Recombination regulator PRDM9 influences the instability of its own coding sequence in humans
- PMID: 23267059
- PMCID: PMC3545772
- DOI: 10.1073/pnas.1220813110
Recombination regulator PRDM9 influences the instability of its own coding sequence in humans
Abstract
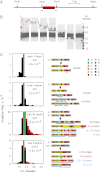
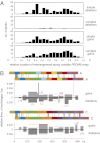
PRDM9 plays a key role in specifying meiotic recombination hotspot locations in humans and mice via recognition of hotspot sequence motifs by a variable tandem-repeat zinc finger domain in the protein. We now explore germ-line instability of this domain in humans. We show that repeat turnover is driven by mitotic and meiotic mutation pathways, the latter frequently resulting in substantial remodeling of zinc fingers. Turnover dynamics predict frequent allele switches in populations with correspondingly fast changes of the recombination landscape, fully consistent with the known rapid evolution of hotspot locations. We found variation in meiotic instability between men that correlated with PRDM9 status. One particular "destabilizer" variant caused hyperinstability not only of itself but also of otherwise-stable alleles in heterozygotes. PRDM9 protein thus appears to regulate the instability of its own coding sequence. However, destabilizer variants are strongly self-limiting in populations and probably have little impact on the evolution of the recombination landscape.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Evolutionary dynamics of meiotic recombination hotspots regulator PRDM9 in bovids.Mol Genet Genomics. 2017 Feb;292(1):117-131. doi: 10.1007/s00438-016-1260-6. Epub 2016 Oct 15. Mol Genet Genomics. 2017. PMID: 27744561
-
Diversity of Prdm9 zinc finger array in wild mice unravels new facets of the evolutionary turnover of this coding minisatellite.PLoS One. 2014 Jan 13;9(1):e85021. doi: 10.1371/journal.pone.0085021. eCollection 2014. PLoS One. 2014. PMID: 24454780 Free PMC article.
-
PRDM9 variation strongly influences recombination hot-spot activity and meiotic instability in humans.Nat Genet. 2010 Oct;42(10):859-63. doi: 10.1038/ng.658. Epub 2010 Sep 5. Nat Genet. 2010. PMID: 20818382 Free PMC article.
-
The case of the fickle fingers: how the PRDM9 zinc finger protein specifies meiotic recombination hotspots in humans.PLoS Biol. 2011 Dec;9(12):e1001211. doi: 10.1371/journal.pbio.1001211. Epub 2011 Dec 6. PLoS Biol. 2011. PMID: 22162947 Free PMC article. Review.
-
The consequences of sequence erosion in the evolution of recombination hotspots.Philos Trans R Soc Lond B Biol Sci. 2017 Dec 19;372(1736):20160462. doi: 10.1098/rstb.2016.0462. Philos Trans R Soc Lond B Biol Sci. 2017. PMID: 29109225 Free PMC article. Review.
Cited by
-
Emergence and influence of sequence bias in evolutionarily malleable, mammalian tandem arrays.BMC Biol. 2023 Aug 23;21(1):179. doi: 10.1186/s12915-023-01673-4. BMC Biol. 2023. PMID: 37612705 Free PMC article.
-
Evolutionary dynamics of meiotic recombination hotspots regulator PRDM9 in bovids.Mol Genet Genomics. 2017 Feb;292(1):117-131. doi: 10.1007/s00438-016-1260-6. Epub 2016 Oct 15. Mol Genet Genomics. 2017. PMID: 27744561
-
The impact of recombination on human mutation load and disease.Philos Trans R Soc Lond B Biol Sci. 2017 Dec 19;372(1736):20160465. doi: 10.1098/rstb.2016.0465. Philos Trans R Soc Lond B Biol Sci. 2017. PMID: 29109227 Free PMC article. Review.
-
Genetic recombination variation in wild Robertsonian mice: on the role of chromosomal fusions and Prdm9 allelic background.Proc Biol Sci. 2014 Jul 7;281(1786):20140297. doi: 10.1098/rspb.2014.0297. Proc Biol Sci. 2014. PMID: 24850922 Free PMC article.
-
Conserved features of recombination control in vertebrates.PLoS Biol. 2025 Jan 7;23(1):e3002959. doi: 10.1371/journal.pbio.3002959. eCollection 2025 Jan. PLoS Biol. 2025. PMID: 39775336 Free PMC article.
References
-
- Kong A, et al. Fine-scale recombination rate differences between sexes, populations and individuals. Nature. 2010;467(7319):1099–1103. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

