Local injection of dsRNA targeting calcitonin receptor-like receptor (CLR) ameliorates Clostridium difficile toxin A-induced ileitis
- PMID: 23267070
- PMCID: PMC3545755
- DOI: 10.1073/pnas.1219733110
Local injection of dsRNA targeting calcitonin receptor-like receptor (CLR) ameliorates Clostridium difficile toxin A-induced ileitis
Erratum in
- Proc Natl Acad Sci U S A. 2013 Feb 26;110(9):3651. Mhsake, Pallavi [corrected to Mhaske, Pallavi]
Abstract
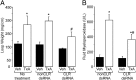
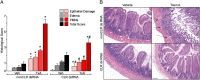
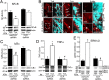
Enteritis caused by Clostridium difficile toxin (Tx) is a nosocomial disease of increasing clinical concern, but the local mediators of C. difficile TxA inflammation are unknown. The potent vasodilator calcitonin gene-related peptide mediates neurogenic inflammation via the calcitonin receptor-like receptor (CLR). Here we examined the ileum-specific effects of reducing CLR on TxA ileitis by local preinjection of double-stranded RNAs. Treatment with CLR dsRNA for 7 d decreased CLR immunoreactivity, whereas treatment with non-CLR dsRNA did not. Subsequent injection of TxA in the same location increased CLR in rats treated with non-CLR dsRNA but not in rats treated with CLR dsRNA, documenting that local injection of dsRNA is effective in preventing the increase in CLR immunoreactivity in response to local TxA. After non-CLR dsRNA pretreatment, TxA induced robust intestinal secretion, myeloperoxidase activity, and histopathologic indications of inflammation including epithelial damage, congestion, neutrophil infiltration, loss of mucin from goblet cells, and increase in mast cell numbers. After CLR dsRNA pretreatment, TxA-induced changes in intestinal secretion and histopathologic inflammation were improved, including normal mucin staining and fewer resident mast cells. Loss of CLR prevented TxA-mediated activation of NF-κB and concomitant increases in pERK1/2 and TNF-α mRNA. Locally produced CLR plays a proinflammatory role in TxA ileitis via MAPK signaling and TNF-α. The results reported here strongly suggest that a local injection of dsRNA targeting CLR could be an effective local therapeutic approach at the inflammation site in the treatment of a growing, clinically relevant hospital-acquired disease, C. difficile infection.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Protease-activated receptor 2, dipeptidyl peptidase I, and proteases mediate Clostridium difficile toxin A enteritis.Gastroenterology. 2007 Jun;132(7):2422-37. doi: 10.1053/j.gastro.2007.03.101. Epub 2007 Apr 13. Gastroenterology. 2007. PMID: 17570216 Free PMC article.
-
Adenosine deaminase inhibition prevents Clostridium difficile toxin A-induced enteritis in mice.Infect Immun. 2011 Feb;79(2):653-62. doi: 10.1128/IAI.01159-10. Epub 2010 Nov 29. Infect Immun. 2011. PMID: 21115723 Free PMC article.
-
Role of calcitonin receptor-like receptor in colonic motility and inflammation.Am J Physiol Gastrointest Liver Physiol. 2007 Jul;293(1):G36-44. doi: 10.1152/ajpgi.00464.2006. Epub 2007 Mar 15. Am J Physiol Gastrointest Liver Physiol. 2007. PMID: 17363466
-
Effects of transcription factor activator protein-1 on interleukin-8 expression and enteritis in response to Clostridium difficile toxin A.J Mol Med (Berl). 2007 Dec;85(12):1393-404. doi: 10.1007/s00109-007-0237-7. Epub 2007 Jul 18. J Mol Med (Berl). 2007. PMID: 17639289
-
Theodore E. Woodward Award. How bacterial enterotoxins work: insights from in vivo studies.Trans Am Clin Climatol Assoc. 2002;113:167-80; discussion 180-1. Trans Am Clin Climatol Assoc. 2002. PMID: 12053708 Free PMC article. Review.
Cited by
-
Fem-1 Gene of Chinese White Pine Beetle (Dendroctonus armandi): Function and Response to Environmental Treatments.Int J Mol Sci. 2024 Sep 26;25(19):10349. doi: 10.3390/ijms251910349. Int J Mol Sci. 2024. PMID: 39408677 Free PMC article.
-
Mitogen-activated protein kinase/IκB kinase/NF-κB-dependent and AP-1-independent CX3CL1 expression in intestinal epithelial cells stimulated with Clostridium difficile toxin A.J Mol Med (Berl). 2014 Apr;92(4):411-27. doi: 10.1007/s00109-013-1117-y. Epub 2013 Dec 22. J Mol Med (Berl). 2014. PMID: 24362517
-
The role of the enteric nervous system in the pathogenesis of Clostridioides difficile infection.Nat Rev Gastroenterol Hepatol. 2025 Aug;22(8):570-586. doi: 10.1038/s41575-025-01071-x. Epub 2025 May 22. Nat Rev Gastroenterol Hepatol. 2025. PMID: 40404838 Review.
-
Constipation Caused by Anti-calcitonin Gene-Related Peptide Migraine Therapeutics Explained by Antagonism of Calcitonin Gene-Related Peptide's Motor-Stimulating and Prosecretory Function in the Intestine.Front Physiol. 2022 Jan 11;12:820006. doi: 10.3389/fphys.2021.820006. eCollection 2021. Front Physiol. 2022. PMID: 35087426 Free PMC article. Review.
-
The Influence of Inflammation and Nerve Damage on the Neurochemical Characterization of Calcitonin Gene-Related Peptide-Like Immunoreactive (CGRP-LI) Neurons in the Enteric Nervous System of the Porcine Descending Colon.Int J Mol Sci. 2018 Feb 12;19(2):548. doi: 10.3390/ijms19020548. Int J Mol Sci. 2018. PMID: 29439512 Free PMC article.
References
-
- Loo VG, et al. Host and pathogen factors for Clostridium difficile infection and colonization. N Engl J Med. 2011;365(18):1693–1703. - PubMed
-
- Pothoulakis C, Lamont JT. Microbes and microbial toxins: Paradigms for microbial-mucosal interactions II. The integrated response of the intestine to Clostridium difficile toxins. Am J Physiol Gastrointest Liver Physiol. 2001;280(2):G178–G183. - PubMed
-
- Pothoulakis C, et al. Substance P receptor expression in intestinal epithelium in Clostridium difficile toxin A enteritis in rats. Am J Physiol. 1998;275(1 Pt 1):G68–G75. - PubMed
-
- Kirkwood KS, et al. Deletion of neutral endopeptidase exacerbates intestinal inflammation induced by Clostridium difficile toxin A. Am J Physiol Gastrointest Liver Physiol. 2001;281(2):G544–G551. - PubMed
-
- Brain SD, Grant AD. Vascular actions of calcitonin gene-related peptide and adrenomedullin. Physiol Rev. 2004;84(3):903–934. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

