Activated protein C ameliorates diabetic nephropathy by epigenetically inhibiting the redox enzyme p66Shc
- PMID: 23267072
- PMCID: PMC3545757
- DOI: 10.1073/pnas.1218667110
Activated protein C ameliorates diabetic nephropathy by epigenetically inhibiting the redox enzyme p66Shc
Abstract
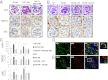
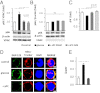
The coagulation protease activated protein C (aPC) confers cytoprotective effects in various in vitro and in vivo disease models, including diabetic nephropathy. The nephroprotective effect may be related to antioxidant effects of aPC. However, the mechanism through which aPC may convey these antioxidant effects and the functional relevance of these properties remain unknown. Here, we show that endogenous and exogenous aPC prevents glomerular accumulation of oxidative stress markers and of the redox-regulating protein p66(Shc) in experimental diabetic nephropathy. These effects were predominately observed in podocytes. In vitro, aPC inhibited glucose-induced expression of p66(Shc) mRNA and protein in podocytes (via PAR-1 and PAR-3) and various endothelial cell lines, but not in glomerular endothelial cells. Treatment with aPC reversed glucose-induced hypomethylation and hyperacetylation of the p66(Shc) promoter in podocytes. The hyperacetylating agent sodium butyrate abolished the suppressive effect of aPC on p66(Shc) expression both in vitro and in vivo. Moreover, sodium butyrate abolished the beneficial effects of aPC in experimental diabetic nephropathy. Inhibition of p66(Shc) expression and mitochondrial translocation by aPC normalized mitochondrial ROS production and the mitochondrial membrane potential in glucose-treated podocytes. Genetic ablation of p66(Shc) compensated for the loss of protein C activation in vivo, normalizing markers of diabetic nephropathy and oxidative stress. These studies identify a unique mechanism underlying the cytoprotective effect of aPC. Activated PC epigenetically controls expression of the redox-regulating protein p66(Shc), thus linking the extracellular protease aPC to mitochondrial function in diabetic nephropathy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Gene silencing of the mitochondrial adaptor p66(Shc) suppresses vascular hyperglycemic memory in diabetes.Circ Res. 2012 Jul 20;111(3):278-89. doi: 10.1161/CIRCRESAHA.112.266593. Epub 2012 Jun 12. Circ Res. 2012. PMID: 22693349
-
Propofol protects against angiotensin II-induced mouse hippocampal HT22 cells apoptosis via inhibition of p66Shc mitochondrial translocation.Neuromolecular Med. 2014 Dec;16(4):772-81. doi: 10.1007/s12017-014-8326-6. Epub 2014 Aug 24. Neuromolecular Med. 2014. PMID: 25151272
-
Deletion of p66Shc longevity gene protects against experimental diabetic glomerulopathy by preventing diabetes-induced oxidative stress.Diabetes. 2006 Jun;55(6):1642-50. doi: 10.2337/db05-1477. Diabetes. 2006. PMID: 16731826
-
p66(Shc) protein, oxidative stress, and cardiovascular complications of diabetes: the missing link.J Mol Med (Berl). 2009 Sep;87(9):885-91. doi: 10.1007/s00109-009-0499-3. Epub 2009 Jul 10. J Mol Med (Berl). 2009. PMID: 19590843 Review.
-
p66(Shc): at the crossroad of oxidative stress and the genetics of aging.Trends Mol Med. 2003 May;9(5):206-10. doi: 10.1016/s1471-4914(03)00048-0. Trends Mol Med. 2003. PMID: 12763525 Review.
Cited by
-
Genetics and Epigenetics of Diabetic Nephropathy.Kidney Dis (Basel). 2015 May;1(1):42-51. doi: 10.1159/000381796. Epub 2015 Apr 16. Kidney Dis (Basel). 2015. PMID: 27536664 Free PMC article. Review.
-
Crosstalk in glomerular injury and repair.Curr Opin Nephrol Hypertens. 2015 May;24(3):231-8. doi: 10.1097/MNH.0000000000000117. Curr Opin Nephrol Hypertens. 2015. PMID: 25887901 Free PMC article. Review.
-
The role and mechanism of butyrate in the prevention and treatment of diabetic kidney disease.Front Microbiol. 2022 Aug 9;13:961536. doi: 10.3389/fmicb.2022.961536. eCollection 2022. Front Microbiol. 2022. PMID: 36016798 Free PMC article. Review.
-
Nlrp3-inflammasome activation in non-myeloid-derived cells aggravates diabetic nephropathy.Kidney Int. 2015 Jan;87(1):74-84. doi: 10.1038/ki.2014.271. Epub 2014 Jul 30. Kidney Int. 2015. PMID: 25075770 Free PMC article.
-
Aerobic Exercise Training Alleviates Renal Injury by Interfering with Mitochondrial Function in Type-1 Diabetic Mice.Med Sci Monit. 2018 Dec 14;24:9081-9089. doi: 10.12659/MSM.912877. Med Sci Monit. 2018. PMID: 30551123 Free PMC article.
References
-
- Riewald M, Petrovan RJ, Donner A, Mueller BM, Ruf W. Activation of endothelial cell protease activated receptor 1 by the protein C pathway. Science. 2002;296(5574):1880–1882. - PubMed
-
- Joyce DE, Gelbert L, Ciaccia A, DeHoff B, Grinnell BW. Gene expression profile of antithrombotic protein c defines new mechanisms modulating inflammation and apoptosis. J Biol Chem. 2001;276(14):11199–11203. - PubMed
-
- Yamaji K, et al. Activated protein C, a natural anticoagulant protein, has antioxidant properties and inhibits lipid peroxidation and advanced glycation end products formation. Thromb Res. 2005;115(4):319–325. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

