Magnetic resonance imaging of blood brain/nerve barrier dysfunction and leukocyte infiltration: closely related or discordant?
- PMID: 23267343
- PMCID: PMC3527731
- DOI: 10.3389/fneur.2012.00178
Magnetic resonance imaging of blood brain/nerve barrier dysfunction and leukocyte infiltration: closely related or discordant?
Abstract
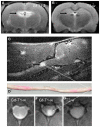
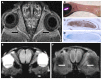
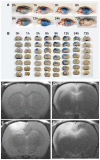
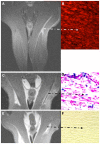
Unlike other organs the nervous system is secluded from the rest of the organism by the blood brain barrier (BBB) or blood nerve barrier (BNB) preventing passive influx of fluids from the circulation. Similarly, leukocyte entry to the nervous system is tightly controlled. Breakdown of these barriers and cellular inflammation are hallmarks of inflammatory as well as ischemic neurological diseases and thus represent potential therapeutic targets. The spatiotemporal relationship between BBB/BNB disruption and leukocyte infiltration has been a matter of debate. We here review contrast-enhanced magnetic resonance imaging (MRI) as a non-invasive tool to depict barrier dysfunction and its relation to macrophage infiltration in the central and peripheral nervous system under pathological conditions. Novel experimental contrast agents like Gadofluorine M (Gf) allow more sensitive assessment of BBB dysfunction than conventional Gadolinium (Gd)-DTPA enhanced MRI. In addition, Gf facilitates visualization of functional and transient alterations of the BBB remote from lesions. Cellular contrast agents such as superparamagnetic iron oxide particles (SPIO) and perfluorocarbons enable assessment of leukocyte (mainly macrophage) infiltration by MR technology. Combined use of these MR contrast agents disclosed that leukocytes can enter the nervous system independent from a disturbance of the BBB, and vice versa, a dysfunctional BBB/BNB by itself is not sufficient to attract inflammatory cells from the circulation. We will illustrate these basic imaging findings in animal models of multiple sclerosis, cerebral ischemia, and traumatic nerve injury and review corresponding findings in patients.
Keywords: blood brain barrier; contrast-enhanced MRI; gadofluorine; gadolinium-DTPA; iron oxide nanoparticles; neuroinflammation.
Figures
References
LinkOut - more resources
Full Text Sources

