ALK receptor activation, ligands and therapeutic targeting in glioblastoma and in other cancers
- PMID: 23267434
- PMCID: PMC3525999
- DOI: 10.3389/fonc.2012.00192
ALK receptor activation, ligands and therapeutic targeting in glioblastoma and in other cancers
Abstract
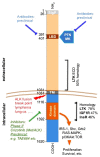
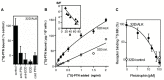
The intracellular anaplastic lymphoma kinase (ALK) fragment shows striking homology with members of the insulin receptor family and was initially identified as an oncogenic fusion protein resulting from a translocation in lymphoma and more recently in a range of cancers. The full-length ALK transmembrane receptor of ~220 kDa was identified based on this initial work. This tyrosine kinase receptor and its ligands, the growth factors pleiotrophin (PTN) and midkine (MK) are highly expressed during development of the nervous system and other organs. Each of these genes has been implicated in malignant progression of different tumor types and shown to alter phenotypes as well as signal transduction in cultured normal and tumor cells. Beyond its role in cancer, the ALK receptor pathway is thought to contribute to nervous system development, function, and repair, as well as metabolic homeostasis and the maintenance of tissue regeneration. ALK receptor activity in cancer can be up-regulated by amplification, overexpression, ligand binding, mutations in the intracellular domain of the receptor and by activity of the receptor tyrosine phosphatase PTPRz. Here we discuss the evidence for ligand control of ALK activity as well as the potential prognostic and therapeutic implications from gene expression and functional studies. An analysis of 18 published gene expression data sets from different cancers shows that overexpression of ALK, its smaller homolog LTK (leukocyte tyrosine kinase) and the ligands PTN and MK in cancer tissues from patients correlate significantly with worse course and outcome of the disease. This observation together with preclinical functional studies suggests that this pathway could be a valid therapeutic target for which complementary targeting strategies with small molecule kinase inhibitors as well as antibodies to ligands or the receptors may be used.
Keywords: anaplastic lymphoma kinase; growth factor; midkine; pleiotrophin; signal transduction.
Figures



References
-
- Bao X., Mikami T., Yamada S., Faissner A., Muramatsu T., Sugahara K. (2005). Heparin-binding growth factor, pleiotrophin, mediates neuritogenic activity of embryonic pig brain-derived chondroitin sulfate/dermatan sulfate hybrid chains. J. Biol. Chem. 280 9180–9191 - PubMed
-
- Bilsland J. G., Wheeldon A., Mead A., Znamenskiy P., Almond S., Waters K. A., et al. (2007). Behavioral and neurochemical alterations in mice deficient in anaplastic lymphoma kinase suggest therapeutic potential for psychiatric indications. Neuropsychopharmacology 33 685–700 - PubMed
-
- Buchholz M., Braun M., Heidenblut A., Kestler H. A., Klöppel G., Schmiegel W., et al. (2005). Transcriptome analysis of microdissected pancreatic intraepithelial neoplastic lesions. Oncogene 24 6626–6636 - PubMed
-
- Chen H., Gordon M. S., Campbell R. A., Li M., Wang C. S., Lee H. J., et al. (2007). Pleiotrophin is highly expressed by myeloma cells and promotes myeloma tumor growth. Blood 110 287–295 - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

