Pharmacokinetics of a single oral dose of vitamin D3 (70,000 IU) in pregnant and non-pregnant women
- PMID: 23268736
- PMCID: PMC3552819
- DOI: 10.1186/1475-2891-11-114
Pharmacokinetics of a single oral dose of vitamin D3 (70,000 IU) in pregnant and non-pregnant women
Abstract
Background: Improvements in antenatal vitamin D status may have maternal-infant health benefits. To inform the design of prenatal vitamin D3 trials, we conducted a pharmacokinetic study of single-dose vitamin D3 supplementation in women of reproductive age.
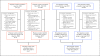
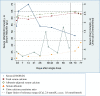
Methods: A single oral vitamin D3 dose (70,000 IU) was administered to 34 non-pregnant and 27 pregnant women (27 to 30 weeks gestation) enrolled in Dhaka, Bangladesh (23°N). The primary pharmacokinetic outcome measure was the change in serum 25-hydroxyvitamin D concentration over time, estimated using model-independent pharmacokinetic parameters.
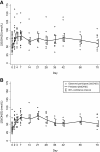
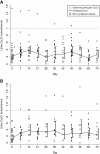
Results: Baseline mean serum 25-hydroxyvitamin D concentration was 54 nmol/L (95% CI 47, 62) in non-pregnant participants and 39 nmol/L (95% CI 34, 45) in pregnant women. Mean peak rise in serum 25-hydroxyvitamin D concentration above baseline was similar in non-pregnant and pregnant women (28 nmol/L and 32 nmol/L, respectively). However, the rate of rise was slightly slower in pregnant women (i.e., lower 25-hydroxyvitamin D on day 2 and higher 25-hydroxyvitamin D on day 21 versus non-pregnant participants). Overall, average 25-hydroxyvitamin D concentration was 19 nmol/L above baseline during the first month. Supplementation did not induce hypercalcemia, and there were no supplement-related adverse events.
Conclusions: The response to a single 70,000 IU dose of vitamin D3 was similar in pregnant and non-pregnant women in Dhaka and consistent with previous studies in non-pregnant adults. These preliminary data support the further investigation of antenatal vitamin D3 regimens involving doses of ≤70,000 IU in regions where maternal-infant vitamin D deficiency is common.
Trial registration: ClinicalTrials.gov NCT00938600.
Figures
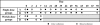

References
-
- Ross AC, Taylor CL, Yaktine AL, Del Valle HB. Committee to Review Dietary Reference Intakes for Vitamin D and Calcium, Institute of Medicine. Dietary Reference Intakes for Calcium and Vitamin D. Washington, DC: The National Academies Press; 2010. - PubMed
-
- Specker BL. Does vitamin D during pregnancy impact offspring growth and bone? Proc Nutr Soc. 2011;FirstView:1–8. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

