Site-specific N-linked glycosylation of receptor guanylyl cyclase C regulates ligand binding, ligand-mediated activation and interaction with vesicular integral membrane protein 36, VIP36
- PMID: 23269669
- PMCID: PMC3567644
- DOI: 10.1074/jbc.M112.413906
Site-specific N-linked glycosylation of receptor guanylyl cyclase C regulates ligand binding, ligand-mediated activation and interaction with vesicular integral membrane protein 36, VIP36
Abstract
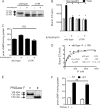
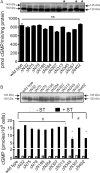
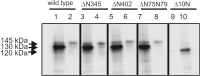
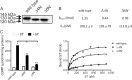
Guanylyl cyclase C (GC-C) is a multidomain, membrane-associated receptor guanylyl cyclase. GC-C is primarily expressed in the gastrointestinal tract, where it mediates fluid-ion homeostasis, intestinal inflammation, and cell proliferation in a cGMP-dependent manner, following activation by its ligands guanylin, uroguanylin, or the heat-stable enterotoxin peptide (ST). GC-C is also expressed in neurons, where it plays a role in satiation and attention deficiency/hyperactive behavior. GC-C is glycosylated in the extracellular domain, and differentially glycosylated forms that are resident in the endoplasmic reticulum (130 kDa) and the plasma membrane (145 kDa) bind the ST peptide with equal affinity. When glycosylation of human GC-C was prevented, either by pharmacological intervention or by mutation of all of the 10 predicted glycosylation sites, ST binding and surface localization was abolished. Systematic mutagenesis of each of the 10 sites of glycosylation in GC-C, either singly or in combination, identified two sites that were critical for ligand binding and two that regulated ST-mediated activation. We also show that GC-C is the first identified receptor client of the lectin chaperone vesicular integral membrane protein, VIP36. Interaction with VIP36 is dependent on glycosylation at the same sites that allow GC-C to fold and bind ligand. Because glycosylation of proteins is altered in many diseases and in a tissue-dependent manner, the activity and/or glycan-mediated interactions of GC-C may have a crucial role to play in its functions in different cell types.
Figures




Similar articles
-
Glycosylation of the receptor guanylate cyclase C: role in ligand binding and catalytic activity.Biochem J. 2004 May 1;379(Pt 3):653-63. doi: 10.1042/BJ20040001. Biochem J. 2004. PMID: 14748740 Free PMC article.
-
Cellular refractoriness to the heat-stable enterotoxin peptide is associated with alterations in levels of the differentially glycosylated forms of guanylyl cyclase C.Eur J Biochem. 2003 Sep;270(18):3848-57. doi: 10.1046/j.1432-1033.2003.03779.x. Eur J Biochem. 2003. PMID: 12950269
-
Expression of the receptor guanylyl cyclase C and its ligands in reproductive tissues of the rat: a potential role for a novel signaling pathway in the epididymis.Biol Reprod. 2002 Dec;67(6):1975-80. doi: 10.1095/biolreprod.102.006445. Biol Reprod. 2002. PMID: 12444076
-
Recognition and signal transduction mechanism of Escherichia coli heat-stable enterotoxin and its receptor, guanylate cyclase C.J Pept Res. 2005 Feb;65(2):261-71. doi: 10.1111/j.1399-3011.2005.00218.x. J Pept Res. 2005. PMID: 15705168 Review.
-
Function and dysfunction of mammalian membrane guanylyl cyclase receptors: lessons from genetic mouse models and implications for human diseases.Handb Exp Pharmacol. 2009;(191):47-69. doi: 10.1007/978-3-540-68964-5_4. Handb Exp Pharmacol. 2009. PMID: 19089325 Review.
Cited by
-
Guanylyl cyclase C as a biomarker for immunotherapies for the treatment of gastrointestinal malignancies.Biomark Med. 2021 Feb;15(3):201-217. doi: 10.2217/bmm-2020-0359. Epub 2021 Jan 20. Biomark Med. 2021. PMID: 33470843 Free PMC article. Review.
-
Congenital secretory diarrhoea caused by activating germline mutations in GUCY2C.Gut. 2016 Aug;65(8):1306-13. doi: 10.1136/gutjnl-2015-309441. Epub 2015 May 20. Gut. 2016. PMID: 25994218 Free PMC article.
-
Biological roles of glycans.Glycobiology. 2017 Jan;27(1):3-49. doi: 10.1093/glycob/cww086. Epub 2016 Aug 24. Glycobiology. 2017. PMID: 27558841 Free PMC article. Review.
-
Rhesus Macaque Killer Cell Ig-like Receptor Domain 0 Glycans Impact Surface Expression and Ligand Specificity.J Immunol. 2024 Dec 15;213(12):1787-1798. doi: 10.4049/jimmunol.2400466. J Immunol. 2024. PMID: 39465971 Free PMC article.
-
N-Glycosylation can selectively block or foster different receptor-ligand binding modes.Sci Rep. 2021 Mar 4;11(1):5239. doi: 10.1038/s41598-021-84569-z. Sci Rep. 2021. PMID: 33664400 Free PMC article.
References
-
- Brown K. H. (2003) Diarrhea and malnutrition. J. Nutr. 133, 328S-332S - PubMed
-
- Hamra F. K., Forte L. R., Eber S. L., Pidhorodeckyj N. V., Krause W. J., Freeman R. H., Chin D. T., Tompkins J. A., Fok K. F., Smith C. E. (1993) Uroguanylin: structure and activity of a second endogenous peptide that stimulates intestinal guanylate cyclase. Proc. Natl. Acad. Sci. U.S.A. 90, 10464–10468 - PMC - PubMed
-
- Basu N., Arshad N., Visweswariah S. S. (2010) Receptor guanylyl cyclase C (GC-C): regulation and signal transduction. Mol Cell Biochem. 334, 67–80 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

