Biophysical and ultrastructural characterization of adeno-associated virus capsid uncoating and genome release
- PMID: 23269804
- PMCID: PMC3592113
- DOI: 10.1128/JVI.03017-12
Biophysical and ultrastructural characterization of adeno-associated virus capsid uncoating and genome release
Abstract
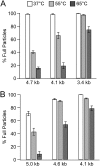
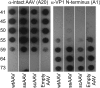
We describe biophysical and ultrastructural differences in genome release from adeno-associated virus (AAV) capsids packaging wild-type DNA, recombinant single-stranded DNA (ssDNA), or dimeric, self-complementary DNA (scDNA) genomes. Atomic force microscopy and electron microscopy (EM) revealed that AAV particles release packaged genomes and undergo marked changes in capsid morphology upon heating in physiological buffer (pH 7.2). When different AAV capsids packaging ss/scDNA varying in length from 72 to 123% of wild-type DNA (3.4 to 5.8 kb) were incrementally heated, the proportion of uncoated AAV capsids decreased with genome length as observed by EM. Genome release was further characterized by a fluorimetric assay, which demonstrated that acidic pH and high osmotic pressure suppress genome release from AAV particles. In addition, fluorimetric analysis corroborated an inverse correlation between packaged genome length and the temperature needed to induce uncoating. Surprisingly, scAAV vectors required significantly higher temperatures to uncoat than their ssDNA-packaging counterparts. However, externalization of VP1 N termini appears to be unaffected by packaged genome length or self-complementarity. Further analysis by tungsten-shadowing EM revealed striking differences in the morphologies of ssDNA and scDNA genomes upon release from intact capsids. Computational modeling and molecular dynamics simulations suggest that the unusual thermal stability of scAAV vectors might arise from partial base pairing and optimal organization of packaged scDNA. Our work further defines the biophysical mechanisms underlying adeno-associated virus uncoating and genome release.
Figures






References
-
- Bowles DE, Rabinowitz JE, Samulski RJ. 2006. The genus Dependovirus, p 15–24 In Kerr JR, Cotmore SF, Bloom ME, Linden RM, Parrish CR. (ed), Parvoviruses. Hodder Arnold Publishers, London, United Kingdom
-
- Samulski RJ, Srivastava A, Berns KI, Muzyczka N. 1983. Rescue of adeno-associated virus from recombinant plasmids: gene correction within the terminal repeats of AAV. Cell 33:135–143 - PubMed
-
- Agbandje-McKenna M, Kleinschmidt J. 2011. AAV capsid structure and cell interactions. Methods Mol. Biol. 807:47–92 - PubMed
-
- Mingozzi F, High KA. 2011. Therapeutic in vivo gene transfer for genetic disease using AAV: progress and challenges. Nat. Rev. Genet. 12:341–355 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

