Neuroligin-1 controls synaptic abundance of NMDA-type glutamate receptors through extracellular coupling
- PMID: 23269831
- PMCID: PMC3545745
- DOI: 10.1073/pnas.1214718110
Neuroligin-1 controls synaptic abundance of NMDA-type glutamate receptors through extracellular coupling
Abstract
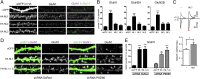
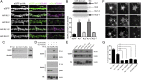
Despite the pivotal functions of the NMDA receptor (NMDAR) for neural circuit development and synaptic plasticity, the molecular mechanisms underlying the dynamics of NMDAR trafficking are poorly understood. The cell adhesion molecule neuroligin-1 (NL1) modifies NMDAR-dependent synaptic transmission and synaptic plasticity, but it is unclear whether NL1 controls synaptic accumulation or function of the receptors. Here, we provide evidence that NL1 regulates the abundance of NMDARs at postsynaptic sites. This function relies on extracellular, NL1 isoform-specific sequences that facilitate biochemical interactions between NL1 and the NMDAR GluN1 subunit. Our work uncovers NL1 isoform-specific cis-interactions with ionotropic glutamate receptors as a key mechanism for controlling synaptic properties.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
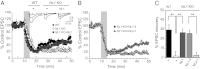


References
-
- Barria A, Malinow R. Subunit-specific NMDA receptor trafficking to synapses. Neuron. 2002;35(2):345–353. - PubMed
-
- Cook EH, Jr, Scherer SW. Copy-number variations associated with neuropsychiatric conditions. Nature. 2008;455(7215):919–923. - PubMed
-
- Lau CG, Zukin RS. NMDA receptor trafficking in synaptic plasticity and neuropsychiatric disorders. Nat Rev Neurosci. 2007;8(6):413–426. - PubMed
-
- Won H, et al. Autistic-like social behaviour in Shank2-mutant mice improved by restoring NMDA receptor function. Nature. 2012;486(7402):261–265. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

