Systematic review and network meta-analysis of combination and monotherapy treatments in disease-modifying antirheumatic drug-experienced patients with rheumatoid arthritis: analysis of American College of Rheumatology criteria scores 20, 50, and 70
- PMID: 23269860
- PMCID: PMC3529627
- DOI: 10.2147/BTT.S36707
Systematic review and network meta-analysis of combination and monotherapy treatments in disease-modifying antirheumatic drug-experienced patients with rheumatoid arthritis: analysis of American College of Rheumatology criteria scores 20, 50, and 70
Abstract
Background: Biologic disease-modifying antirheumatic drugs (bDMARDs) extend the treatment choices for rheumatoid arthritis patients with suboptimal response or intolerance to conventional DMARDs. The objective of this systematic review and meta-analysis was to compare the relative efficacy of EU-licensed bDMARD combination therapy or monotherapy for patients intolerant of or contraindicated to continued methotrexate.
Methods: Comprehensive, structured literature searches were conducted in Medline, Embase, and the Cochrane Library, as well as hand-searching of conference proceedings and reference lists. Phase II or III randomized controlled trials reporting American College of Rheumatology (ACR) criteria scores of 20, 50, and 70 between 12 and 30 weeks' follow-up and enrolling adult patients meeting ACR classification criteria for rheumatoid arthritis previously treated with and with an inadequate response to conventional DMARDs were eligible. To estimate the relative efficacy of treatments whilst preserving the randomized comparisons within each trial, a Bayesian network meta-analysis was conducted in WinBUGS using fixed and random-effects, logit-link models fitted to the binomial ACR 20/50/70 trial data.
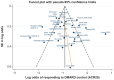
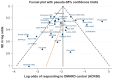
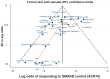
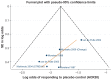
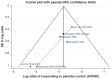
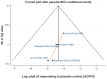
Results: The systematic review identified 10,625 citations, and after a review of 2450 full-text papers, there were 29 and 14 eligible studies for the combination and monotherapy meta-analyses, respectively. In the combination analysis, all licensed bDMARD combinations had significantly higher odds of ACR 20/50/70 compared to DMARDs alone, except for the rituximab comparison, which did not reach significance for the ACR 70 outcome (based on the 95% credible interval). The etanercept combination was significantly better than the tumor necrosis factor-α inhibitors adalimumab and infliximab in improving ACR 20/50/70 outcomes, with no significant differences between the etanercept combination and certolizumab pegol or tocilizumab. Licensed-dose etanercept, adalimumab, and tocilizumab monotherapy were significantly better than placebo in improving ACR 20/50/70 outcomes. Sensitivity analysis indicated that including studies outside the target population could affect the results.
Conclusion: Licensed bDMARDs are efficacious in patients with an inadequate response to conventional therapy, but tumor necrosis factor-α inhibitor combination therapies are not equally effective.
Keywords: bDMARD; comparative effectiveness; etanercept; network metaanalysis; rheumatoid arthritis; systematic review.
Figures


References
-
- Lee DM, Weinblatt ME. Rheumatoid arthritis. Lancet. 2001;358(9285):903–911. - PubMed
-
- Symmons D, Turner G, Webb R, et al. The prevalence of rheumatoid arthritis in the United Kingdom: new estimates for a new century. Rheumatology (Oxford) 2002;41(7):793–800. - PubMed
-
- Smith HS, Smith AR, Seidner P. Painful rheumatoid arthritis. Pain Physician. 2011;14(5):E427–E458. - PubMed
LinkOut - more resources
Full Text Sources

