Phase II study of lenalidomide and rituximab as salvage therapy for patients with relapsed or refractory chronic lymphocytic leukemia
- PMID: 23270003
- PMCID: PMC4878047
- DOI: 10.1200/JCO.2012.42.8623
Phase II study of lenalidomide and rituximab as salvage therapy for patients with relapsed or refractory chronic lymphocytic leukemia
Abstract
Purpose: Lenalidomide is an immunomodulatory drug active as salvage therapy for chronic lymphocytic leukemia (CLL). We combined lenalidomide with rituximab to improve response rates in patients with relapsed or refractory CLL.
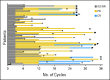
Patients and methods: Fifty-nine adult patients (age 42 to 82 years) with relapsed or refractory CLL were enrolled onto a phase II study of lenalidomide and rituximab. Patients had received prior fludarabine-based therapy or chemoimmunotherapy. Rituximab (375 mg/m(2) intravenously) was administered weekly during cycle one and on day 1 of cycles three to 12. Lenalidomide was started on day 9 of cycle one at 10 mg orally and administered daily continuously. Each cycle was 28 days. Rituximab was administered for 12 cycles; lenalidomide could continue indefinitely if patients benefitted clinically.
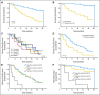
Results: The overall response rate was 66%, including 12% complete responses and 12% nodular partial remissions. Time to treatment failure was 17.4 months. Median overall survival has not been reached; estimated survival at 36 months is 71%. The most common grade 3 or 4 toxicity was neutropenia (73% of patients). Fourteen patients (24%) experienced a grade 3 to 4 infection or febrile episode. There was one episode of grade 3 tumor lysis; one patient experienced renal failure during the first cycle of therapy, and one venous thromboembolic event occurred during the study.
Conclusion: The combination of lenalidomide and rituximab is active in patients with recurrent CLL and warrants further investigation.
Trial registration: ClinicalTrials.gov NCT00759603.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures
Similar articles
-
Lenalidomide and rituximab for the initial treatment of patients with chronic lymphocytic leukemia: a multicenter clinical-translational study from the chronic lymphocytic leukemia research consortium.J Clin Oncol. 2014 Jul 1;32(19):2067-73. doi: 10.1200/JCO.2013.51.5890. Epub 2014 May 27. J Clin Oncol. 2014. PMID: 24868031 Free PMC article.
-
Results of a phase II study of lenalidomide and rituximab for refractory/relapsed chronic lymphocytic leukemia.Leuk Res. 2016 Aug;47:78-83. doi: 10.1016/j.leukres.2016.05.012. Epub 2016 May 17. Leuk Res. 2016. PMID: 27285853 Clinical Trial.
-
Fludarabine and rituximab with escalating doses of lenalidomide followed by lenalidomide/rituximab maintenance in previously untreated chronic lymphocytic leukaemia (CLL): the REVLIRIT CLL-5 AGMT phase I/II study.Ann Hematol. 2018 Oct;97(10):1825-1839. doi: 10.1007/s00277-018-3380-z. Epub 2018 Jun 4. Ann Hematol. 2018. PMID: 29862437 Free PMC article. Clinical Trial.
-
Rituximab: a review of its use in non-Hodgkin's lymphoma and chronic lymphocytic leukaemia.Drugs. 2003;63(8):803-43. doi: 10.2165/00003495-200363080-00005. Drugs. 2003. PMID: 12662126 Review.
-
Lenalidomide and chronic lymphocytic leukemia.Biomed Res Int. 2013;2013:932010. doi: 10.1155/2013/932010. Epub 2013 Sep 19. Biomed Res Int. 2013. PMID: 24163824 Free PMC article. Review.
Cited by
-
Immunomodulatory Drugs for the Treatment of B Cell Malignancies.Int J Mol Sci. 2021 Aug 9;22(16):8572. doi: 10.3390/ijms22168572. Int J Mol Sci. 2021. PMID: 34445275 Free PMC article. Review.
-
T Cell Defects and Immunotherapy in Chronic Lymphocytic Leukemia.Cancers (Basel). 2021 Jun 29;13(13):3255. doi: 10.3390/cancers13133255. Cancers (Basel). 2021. PMID: 34209724 Free PMC article. Review.
-
Clinical outcomes of a novel combination of lenalidomide and rituximab followed by stem cell transplantation for relapsed/refractory aggressive B-cell non-hodgkin lymphoma.Oncotarget. 2014 Sep 15;5(17):7368-80. doi: 10.18632/oncotarget.2255. Oncotarget. 2014. PMID: 25228589 Free PMC article. Clinical Trial.
-
Immunomodulatory Drugs in Multiple Myeloma: Mechanisms of Action and Clinical Experience.Drugs. 2017 Apr;77(5):505-520. doi: 10.1007/s40265-017-0689-1. Drugs. 2017. PMID: 28205024 Free PMC article. Review.
-
A phase 1 study of lenalidomide and ibrutinib in combination with rituximab in relapsed and refractory CLL.Blood Adv. 2018 Apr 10;2(7):762-768. doi: 10.1182/bloodadvances.2017015263. Blood Adv. 2018. PMID: 29610115 Free PMC article. Clinical Trial.
References
-
- Hallek M, Fischer K, Fingerle-Rowson G, et al. Addition of rituximab to fludarabine and cyclophosphamide in patients with chronic lymphocytic leukaemia: A randomised, open-label, phase 3 trial. Lancet. 2010;376:1164–1174. - PubMed
-
- Bergmann MA, Goebeler ME, Herold M, et al. Efficacy of bendamustine in patients with relapsed or refractory chronic lymphocytic leukemia: Results of a phase I/II study of the German CLL Study Group. Haematologica. 2005;90:1357–1364. - PubMed
-
- Iannitto E, Morabito F, Mancuso S, et al. Bendamustine with or without rituximab in the treatment of relapsed chronic lymphocytic leukaemia: An Italian retrospective study. Br J Haematol. 2011;153:351–357. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

