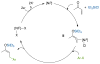
Nickel-catalyzed reductive conjugate addition to enones via allylnickel intermediates
- PMID: 23270480
- PMCID: PMC3547151
- DOI: 10.1021/ja309176h
Nickel-catalyzed reductive conjugate addition to enones via allylnickel intermediates
Abstract
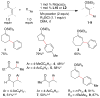
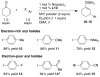
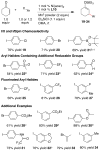
An alternative method to copper-catalyzed conjugate addition followed by enolate silylation for the synthesis of β-disubstituted silyl enol ether products (R(1)(R(2))HCCH═C(OSiR(4)(3))R(3)) is presented. This method uses haloarenes instead of nucleophilic aryl reagents. Nickel ligated to either neocuproine or bipyridine couples an α,β-unsaturated ketone or aldehyde (R(2)HC═CHC(O)R(3)) with an organic halide (R(1)-X) in the presence of a trialkylchlorosilane reagent (Cl-SiR(4)(3)). Reactions are assembled on the benchtop and tolerate a variety of functional groups (aldehyde, ketone, nitrile, sulfone, pentafluorosulfur, and N-aryltrifluoroacetamide), electron-rich iodoarenes, and electron-poor haloarenes. Mechanistic studies have confirmed the first example of a catalytic reductive conjugate addition of organic halides that proceeds via an allylnickel intermediate. Selectivity is attributed to (1) rapid, selective reaction of LNi(0) with chlorotriethylsilane and enone in the presence of other organic electrophiles, and (2) minimization of enone dimerization by ligand steric effects.
Figures


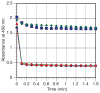
 ), cyclohexenone + Et3SiCl (
), cyclohexenone + Et3SiCl (
 ), Et3SiCl (●), and cyclohexenone (
), Et3SiCl (●), and cyclohexenone (
 ) as monitored by UV-Vis at 450 nm. For full UV-Vis spectra and expanded plots of all four reactions, see Figures S1–S3 in the Supporting Information.
) as monitored by UV-Vis at 450 nm. For full UV-Vis spectra and expanded plots of all four reactions, see Figures S1–S3 in the Supporting Information.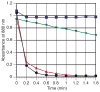
 ), cyclohexenone + Et3SiCl (
), cyclohexenone + Et3SiCl (
 ), Et3SiCl (●), and cyclohexenone (
), Et3SiCl (●), and cyclohexenone (
 ) as monitored by UV-Vis at 880 nm. For full UV-Vis spectra and an expanded plot of all four reactions, see Figures S11 and S12 in the Supporting Information.
) as monitored by UV-Vis at 880 nm. For full UV-Vis spectra and an expanded plot of all four reactions, see Figures S11 and S12 in the Supporting Information.

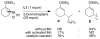
References
-
- Kharasch MS, Tawney PO. J Am Chem Soc. 1941;63:2308.
- Perlmutter P. Conjugate Addition Reactions in Organic Synthesis. Pergamon Press; Oxford: 1992.
- Taylor RJK. Synthesis. 1985:364.
- Nakamura E. Synlett. 1991:539.
- Krause N, Gerold A. Angew Chem, Int Ed. 1997;36:186.
- Howell GP. Org Process Res Dev. 2012;16:1258.
-
- Kobayashi S, Manabe K, Ishitani H, Matsuo J-I. Sci Synth. 2002;4:317.
- Stork G, Hudrlik PF. J Am Chem Soc. 1968;90:4464.
- Ruecker C. Chem Rev. 1995;95:1009.
-
-
Trialkylchlorosilane reagents accelerate copper-mediated conjugate addition reactions. Lipshutz BH, Dimock SH, James B. J Am Chem Soc. 1993;115:9283.Frantz DE, Singleton DA. J Am Chem Soc. 2000;122:3288.
-
-
- Högermeier J, Reissig HU. Adv Synth Catal. 2009;351:2747–2763.
-
- Kuwajima I, Urabe H. J Am Chem Soc. 1982;104:6831.
- Su W, Raders S, Verkade JG, Liao X, Hartwig JF. Angew Chem, Int Ed. 2006;45:5852. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

