Patient-reported outcomes to assess the efficacy of extended-release guaifenesin for the treatment of acute respiratory tract infection symptoms
- PMID: 23270519
- PMCID: PMC3546880
- DOI: 10.1186/1465-9921-13-118
Patient-reported outcomes to assess the efficacy of extended-release guaifenesin for the treatment of acute respiratory tract infection symptoms
Abstract
Background: Guaifenesin is a component of medicines used to improve symptoms associated with upper respiratory tract infections. Patient-reported outcome instruments are valuable for evaluating symptom improvements; however, a validated tool to assess efficacy of mucoactive drugs does not exist. We compared the efficacy of extended-release guaifenesin with placebo for treatment of symptoms of upper respiratory tract infection using subjective efficacy assessments in a pilot study and confirmed precision of assessments in a validation study.
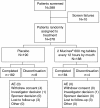
Methods: The pilot study was a randomized, double-blind study where patients were dosed with either 1200 mg extended-release guaifenesin (n = 188) or placebo (n = 190), every 12 hours for 7 days. Efficacy was assessed using subjective measures including the Daily Cough and Phlegm Diary, the Spontaneous Symptom Severity Assessment and the Wisconsin Upper Respiratory Symptom Survey. End-of-study assessments were completed by patients and investigator. The validation study consisted of two phases. In Phase I, subjects completed interviews to gather evidence to support the content validity of the Daily Cough and Phlegm Diary, the Spontaneous Symptom Severity Assessment and Patient's End-of-Treatment Assessment. Phase II examined the psychometric properties of assessments evaluated in Phase I of the validation study using data from the pilot study.
Results: Subjective measures of efficacy at Day 4 showed the most prominent difference between treatment groups, in favor of guaifenesin. The 8-symptom related questions (SUM8) in the Daily Cough and Phlegm Diary, analyzed as a composite score appeared to be the strongest candidate endpoint for further evaluation. Results from the interviews in Phase I supported the content of the assessments which were validated during Phase II. Treatments were well tolerated.
Conclusions: Results from the clinical pilot and validation studies showed that the SUM8 diary scores were robust and reliable for use as efficacy endpoints in studies of mucoactive drugs.
Trial registration: The study was registered with clinicaltrials.gov (NCT01046136).
Figures
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical