Implementation of filmarray respiratory viral panel in a core laboratory improves testing turnaround time and patient care
- PMID: 23270907
- PMCID: PMC7110182
- DOI: 10.1309/AJCPH7X3NLYZPHBW
Implementation of filmarray respiratory viral panel in a core laboratory improves testing turnaround time and patient care
Abstract
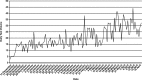
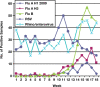
The FilmArray respiratory virus panel detects 15 viral agents in respiratory specimens using polymerase chain reaction. We performed FilmArray respiratory viral testing in a core laboratory at a regional children's hospital that provides service 24 hours a day 7 days a week. The average and median turnaround time were 1.6 and 1.4 hours, respectively, in contrast to 7 and 6.5 hours documented 1 year previously at an on-site reference laboratory using a direct fluorescence assay (DFA) that detected 8 viral agents. During the study period, rhinovirus was detected in 20% and coronavirus in 6% of samples using FilmArray; these viruses would not have been detected with DFA. We followed 97 patients with influenza A or influenza B who received care at the emergency department (ED). Overall, 79 patients (81%) were given oseltamivir in a timely manner defined as receiving the drug in the ED, a prescription in the ED, or a prescription within 3 hours of ED discharge. Our results demonstrate that molecular technology can be successfully deployed in a nonspecialty, high-volume, multidisciplinary core laboratory.
Figures
References
-
- Chapin K. Multiplex PCR for detection of respiratory viruses: can the laboratory performing a respiratory viral panel (RVP) assay trigger better patient care and clinical outcomes? Clin Biochem. 2011;44:496–497. - PubMed
-
- Byington CL, Castillo H, Gerber K, et al. The effect of rapid respiratory viral diagnostic testing on antibiotic use in a children’s hospital. Arch Pediatr Adolesc Med. 2002;156:1230–1234. - PubMed
-
- Miernyk K, Bulkow L, DeByle C, et al. Performance of a rapid antigen test (Binax NOW® RSV) for diagnosis of respiratory syncytial virus compared with real-time polymerase chain reaction in a pediatric population. J Clin Virol. 2011;50:240–243. - PubMed
-
- Ganzenmueller T, Kluba J, Hilfrich B, et al. Comparison of the performance of direct fluorescent antibody staining, a point-of-care rapid antigen test and virus isolation with that of RT-PCR for the detection of novel 2009 influenza A (H1N1) virus in respiratory specimens. J Med Microbiol. 2010;59:713–717. - PubMed
-
- Takahashi H, Otsuka Y, Patterson BK. Diagnostic tests for influenza and other respiratory viruses: determining performance specifications based on clinical setting. J Infect Chemother. 2010;16:155–161. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

