Silk-based injectable biomaterial as an alternative to cervical cerclage: an in vitro study
- PMID: 23271162
- PMCID: PMC3713644
- DOI: 10.1177/1933719112468952
Silk-based injectable biomaterial as an alternative to cervical cerclage: an in vitro study
Abstract
Objective: New therapies to prevent preterm birth are needed. Our objective was to study an injectable biomaterial for human cervical tissue as an alternative to cervical cerclage.
Study design: Human cervical tissue specimens were obtained from premenopausal gynecological hysterectomies for benign indications. A 3-part biomaterial was formulated, consisting of silk protein solution blended with a 2-part polyethylene glycol gelation system. The solutions were injected into cervical tissue and the tissue was evaluated for mechanical properties, swelling, cytocompatibility, and histology.
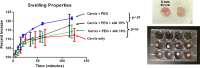
Results: The stiffness of cervical tissue more than doubled after injection (P = .02). Swelling properties of injected tissue were no different than native tissue controls. Cervical fibroblasts remained viable for at least 48 hours when cultured on the biomaterial.
Conclusions: We report a silk-based, biocompatible, injectable biomaterial that increased the stiffness of cervical tissue compared to uninjected controls. Animal studies are needed to assess this biomaterial in vivo.
Keywords: cerclage; cervix; injectable biomaterial; preterm birth; silk.
Conflict of interest statement
Figures



Similar articles
-
Biocompatibility of a sonicated silk gel for cervical injection during pregnancy: in vivo and in vitro study.Reprod Sci. 2014 Oct;21(10):1266-73. doi: 10.1177/1933719114522551. Epub 2014 Feb 11. Reprod Sci. 2014. PMID: 24520079 Free PMC article.
-
Injectable Silk-Based Hydrogel as an Alternative to Cervical Cerclage: A Rabbit Study.Tissue Eng Part A. 2020 Apr;26(7-8):379-386. doi: 10.1089/ten.TEA.2019.0210. Epub 2019 Nov 14. Tissue Eng Part A. 2020. PMID: 31621512 Free PMC article.
-
Injectable silk-based biomaterials for cervical tissue augmentation: an in vitro study.Am J Obstet Gynecol. 2016 Jan;214(1):118.e1-9. doi: 10.1016/j.ajog.2015.08.046. Epub 2015 Aug 24. Am J Obstet Gynecol. 2016. PMID: 26314518 Free PMC article.
-
Cervical cerclage in the prevention of preterm birth.Best Pract Res Clin Obstet Gynaecol. 2007 Oct;21(5):831-42. doi: 10.1016/j.bpobgyn.2007.03.009. Epub 2007 May 10. Best Pract Res Clin Obstet Gynaecol. 2007. PMID: 17493875 Review.
-
Cervical cerclage for the prevention of preterm birth.Obstet Gynecol Clin North Am. 2012 Mar;39(1):25-33. doi: 10.1016/j.ogc.2011.12.001. Epub 2012 Jan 4. Obstet Gynecol Clin North Am. 2012. PMID: 22370105 Review.
Cited by
-
Ex vivo pregnant-like tissue model to assess injectable hydrogel for preterm birth prevention.J Biomed Mater Res B Appl Biomater. 2020 Feb;108(2):468-474. doi: 10.1002/jbm.b.34403. Epub 2019 May 9. J Biomed Mater Res B Appl Biomater. 2020. PMID: 31070848 Free PMC article.
-
Tissue Engineering for Cervical Function in Pregnancy.Curr Opin Biomed Eng. 2022 Jun;22:100385. doi: 10.1016/j.cobme.2022.100385. Epub 2022 Mar 28. Curr Opin Biomed Eng. 2022. PMID: 35574159 Free PMC article.
-
Cervical Augmentation with an Injectable Silk-Based Gel: Biocompatibility in a Rat Model of Pregnancy.Reprod Sci. 2020 May;27(5):1215-1221. doi: 10.1007/s43032-019-00111-7. Epub 2020 Jan 6. Reprod Sci. 2020. PMID: 32046447 Free PMC article.
-
Biocompatibility of a sonicated silk gel for cervical injection during pregnancy: in vivo and in vitro study.Reprod Sci. 2014 Oct;21(10):1266-73. doi: 10.1177/1933719114522551. Epub 2014 Feb 11. Reprod Sci. 2014. PMID: 24520079 Free PMC article.
-
Injectable Silk-Based Hydrogel as an Alternative to Cervical Cerclage: A Rabbit Study.Tissue Eng Part A. 2020 Apr;26(7-8):379-386. doi: 10.1089/ten.TEA.2019.0210. Epub 2019 Nov 14. Tissue Eng Part A. 2020. PMID: 31621512 Free PMC article.
References
-
- Martin JA, Hamilton BE, Sutton PD, et al. Births: final data for 2007. Natl Vital Stat Rep. 2010;58(24):1–85. - PubMed
-
- Institute of Medicine. Preterm Birth: Causes, Consequences, and Prevention. Washington: DC: National Academies Press; 2006.
-
- Mathews TJ, MacDorman MF. Infant mortality statistics from the 2006 period linked birth/infant death data set. Natl Vital Stat Rep. 2010; 58(17):1–31. - PubMed
-
- Saigal S, Doyle LW. An overview of mortality and sequelae of preterm birth from infancy to adulthood. Lancet. 2008; 371(9608):261–269. - PubMed
-
- Iams JD, Goldenberg RL, Meis PJ, et al. The length of the cervix and the risk of spontaneous premature delivery. N Engl J Med. 1996; 334(9):567–572. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

