Distribution of red-spotted grouper nervous necrosis virus (RGNNV) antigens in nervous and non-nervous organs of European seabass(Dicentrarchus labrax) during the course of an experimental challenge
- PMID: 23271176
- PMCID: PMC3539120
- DOI: 10.4142/jvs.2012.13.4.355
Distribution of red-spotted grouper nervous necrosis virus (RGNNV) antigens in nervous and non-nervous organs of European seabass(Dicentrarchus labrax) during the course of an experimental challenge
Abstract
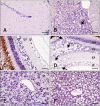
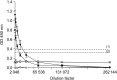
The distribution of red-spotted grouper nervous necrosis virus (RGNNV) antigens was examined by immunohistochemistry in the nervous and non-nervous organs of juvenile European seabass (Dicentrarchus labrax) during the course of an intramuscular infection. Histological changes resulting from the infection were evaluated from 3 days to 2 months post-infection. The specific antibody response was also studied 2 months post-challenge. Viral proteins were present throughout the experimental period in the retina (inner nuclear layer, ganglion layer, outer limiting membrane, and outer plexiform layer), brain(cerebellum and tectum opticum), and liver (hepatocytes and endothelial cells). These proteins were also observed in the renal tubular cells, white pulp of spleen, and in fibroblasts and cartilage of caudal fin. This is the first report of RGNNV proteins appearing in these organs, where the immunostaining was only detected at certain sampling times after the onset of mortality. Brain and retina of virus-exposed fish showed high levels of vacuolation, while accumulation of fat vacuoles was observed in the liver. RGNNV infection also induced a specific antibody response as measured by an ELISA. In summary, this is the first study demonstrating the presence of viral proteins in cells of caudal fin, kidney and spleen of European seabass.
Figures


Similar articles
-
Tissue distribution of Red Spotted Grouper Nervous Necrosis Virus (RGNNV) genome in experimentally infected juvenile European seabass (Dicentrarchus labrax).Vet Microbiol. 2011 Dec 29;154(1-2):86-95. doi: 10.1016/j.vetmic.2011.06.029. Epub 2011 Jul 1. Vet Microbiol. 2011. PMID: 21783331
-
Detection of Betanodavirus in experimentally infected European seabass (Dicentrarchus labrax, Linnaeus 1758) using non-lethal sampling methods.J Fish Dis. 2019 Aug;42(8):1097-1105. doi: 10.1111/jfd.13015. Epub 2019 Jun 10. J Fish Dis. 2019. PMID: 31180142
-
Characterization of microRNAs in orange-spotted grouper (Epinephelus coioides) fin cells upon red-spotted grouper nervous necrosis virus infection.Fish Shellfish Immunol. 2017 Apr;63:228-236. doi: 10.1016/j.fsi.2017.02.031. Epub 2017 Feb 20. Fish Shellfish Immunol. 2017. PMID: 28232192
-
Investigation of routes of entry and dispersal pattern of RGNNV in tissues of European sea bass, Dicentrarchus labrax.J Fish Dis. 2020 Nov;43(11):1363-1371. doi: 10.1111/jfd.13215. Epub 2020 Sep 3. J Fish Dis. 2020. PMID: 32882747
-
Lack of in vivo cross-protection of two different betanodavirus species RGNNV and SJNNV in European sea bass Dicentrachus labrax.Fish Shellfish Immunol. 2019 Feb;85:85-89. doi: 10.1016/j.fsi.2017.10.033. Epub 2017 Oct 19. Fish Shellfish Immunol. 2019. PMID: 29056488
Cited by
-
Characterization of Hagfish (Eptatretus burgeri) Variable Lymphocyte Receptor-Based Antibody and Its Potential Role in the Neutralization of Nervous Necrosis Virus.J Immunol. 2020 Feb 1;204(3):718-725. doi: 10.4049/jimmunol.1900675. Epub 2019 Dec 13. J Immunol. 2020. PMID: 31836656 Free PMC article.
-
Betanodavirus and VER Disease: A 30-year Research Review.Pathogens. 2020 Feb 9;9(2):106. doi: 10.3390/pathogens9020106. Pathogens. 2020. PMID: 32050492 Free PMC article. Review.
-
Elucidating the Functional Roles of Helper and Cytotoxic T Cells in the Cell-Mediated Immune Responses of Olive Flounder (Paralichthys olivaceus).Int J Mol Sci. 2021 Jan 15;22(2):847. doi: 10.3390/ijms22020847. Int J Mol Sci. 2021. PMID: 33467734 Free PMC article.
-
Development of a New Marine Fish Continuous Cell Line Derived from Brain of Red Sea Bream (Pagrosomus major) and Its Application to Fish Virology and Heavy Metal Toxicology.Animals (Basel). 2023 Nov 15;13(22):3524. doi: 10.3390/ani13223524. Animals (Basel). 2023. PMID: 38003142 Free PMC article.
-
Capsid amino acids at positions 247 and 270 are involved in the virulence of betanodaviruses to European sea bass.Sci Rep. 2019 Oct 1;9(1):14068. doi: 10.1038/s41598-019-50622-1. Sci Rep. 2019. PMID: 31575937 Free PMC article.
References
-
- Alonso MC, Cano I, Garcia-Rosado E, Castro D, Lamas J, Barja JL, Borrego JJ, Bergmann SM. Isolation of lymphocystis disease virus from sole, Solea senegalensis Kaup, and blackspot sea bream, Pagellus bogaraveo (Brünnich) J Fish Dis. 2005;28:221–228. - PubMed
-
- Aranguren R, Tafalla C, Novoa B, Figueras A. Experimental transmission of encephalopathy and retinopathy induced by nodavirus to sea bream, Sparus aurata L., using different infection models. J Fish Dis. 2002;25:317–324.
-
- Athanassopoulou F, Billinis C, Psychas V, Karipoglou K. Viral encephalopathy and retinopathy of Dicentrarchus labrax (L.) farmed in fresh water in Greece. J Fish Dis. 2003;26:361–365. - PubMed
-
- Azad IS, Shekhar MS, Thirunavukkarasu AR, Jithendran KP. Viral nerve necrosis in hatchery-produced fry of Asian seabass Lates calcarifer: sequential microscopic analysis of histopathology. Dis Aquat Organ. 2006;73:123–130. - PubMed
-
- Bigarré L, Baud M, Cabon J, Crenn K, Castric J. New PCR probes for detection and genotyping of piscine betanodaviruses. J Fish Dis. 2010;33:907–912. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

