Chiral peptide nucleic acids with a substituent in the N-(2-aminoethy)glycine backbone
- PMID: 23271467
- PMCID: PMC6269907
- DOI: 10.3390/molecules18010287
Chiral peptide nucleic acids with a substituent in the N-(2-aminoethy)glycine backbone
Abstract
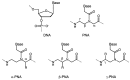
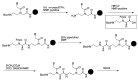
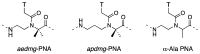
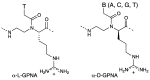
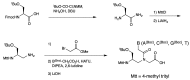
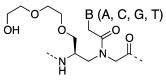
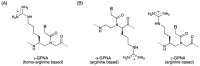
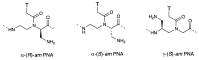
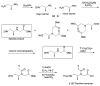
A peptide nucleic acid (PNA) is a synthetic nucleic acid mimic in which the sugar-phosphate backbone is replaced by a peptide backbone. PNAs hybridize to complementary DNA and RNA with higher affinity and superior sequence selectivity compared to DNA. PNAs are resistant to nucleases and proteases and have a low affinity for proteins. These properties make PNAs an attractive agent for biological and medical applications. To improve the antisense and antigene properties of PNAs, many backbone modifications of PNAs have been explored under the concept of preorganization. This review focuses on chiral PNAs bearing a substituent in the N-(2-aminoethyl)glycine backbone. Syntheses, properties, and applications of chiral PNAs are described.
Figures

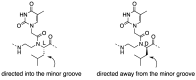



Similar articles
-
Peptide nucleic acids: Advanced tools for biomedical applications.J Biotechnol. 2017 Oct 10;259:148-159. doi: 10.1016/j.jbiotec.2017.07.026. Epub 2017 Jul 29. J Biotechnol. 2017. PMID: 28764969 Free PMC article. Review.
-
Peptide-nucleic acids (PNAs): a tool for the development of gene expression modifiers.Curr Pharm Des. 2001 Nov;7(17):1839-62. doi: 10.2174/1381612013397087. Curr Pharm Des. 2001. PMID: 11562312 Review.
-
[The peptide nucleic acids (PNAs): "high-tech" probes for genetic and molecular cytogenetic investigations].Med Sci (Paris). 2005 Aug-Sep;21(8-9):753-8. doi: 10.1051/medsci/2005218-9753. Med Sci (Paris). 2005. PMID: 16115462 Review. French.
-
Side chain modified peptide nucleic acids (PNA) for knock-down of six3 in medaka embryos.BMC Biotechnol. 2012 Aug 17;12:50. doi: 10.1186/1472-6750-12-50. BMC Biotechnol. 2012. PMID: 22901024 Free PMC article.
-
Peptide nucleic acid-DNA decoy chimeras targeting NF-kappaB transcription factors: Induction of apoptosis in human primary osteoclasts.Int J Mol Med. 2004 Aug;14(2):145-52. Int J Mol Med. 2004. PMID: 15254756
Cited by
-
Peptide nucleic acids: Advanced tools for biomedical applications.J Biotechnol. 2017 Oct 10;259:148-159. doi: 10.1016/j.jbiotec.2017.07.026. Epub 2017 Jul 29. J Biotechnol. 2017. PMID: 28764969 Free PMC article. Review.
-
Special Issue: Molecular Properties and the Applications of Peptide Nucleic Acids.Molecules. 2018 Aug 8;23(8):1977. doi: 10.3390/molecules23081977. Molecules. 2018. PMID: 30096770 Free PMC article.
-
Polyanionic Carboxyethyl Peptide Nucleic Acids (ce-PNAs): Synthesis and DNA Binding.PLoS One. 2015 Oct 15;10(10):e0140468. doi: 10.1371/journal.pone.0140468. eCollection 2015. PLoS One. 2015. PMID: 26469337 Free PMC article.
-
Perspectives on conformationally constrained peptide nucleic acid (PNA): insights into the structural design, properties and applications.RSC Chem Biol. 2022 Mar 18;3(6):648-697. doi: 10.1039/d2cb00017b. eCollection 2022 Jun 8. RSC Chem Biol. 2022. PMID: 35755191 Free PMC article. Review.
-
Facile synthesis of new N-(aminocycloalkylene)amino acid compounds using chiral triflate esters with N-Boc-aminopyrrolidines and N-Boc-aminopiperidines.RSC Adv. 2023 Jul 18;13(31):21378-21394. doi: 10.1039/d3ra03060a. eCollection 2023 Jul 12. RSC Adv. 2023. PMID: 37469966 Free PMC article.
References
-
- Nielsen P.E., Egholm M., Berg R.H., Buchardt O. Sequence-selective recognition of DNA by strand displacement with a thymine-substituted polyamide. Science. 1991;254:1497–1500. - PubMed
-
- Egholm M., Buchardt O., Christensen L., Behrens C., Freier S.M., Driver D.A., Berg R.H., Kim S.K., Norden B., Nielsen P.E. PNA hybridizes to complementary oligonucleotides obeying the Watson–Crick hydrogen-bonding rules. Nature. 1993;365:566–568. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

