Activity of the mitochondrial calcium uniporter varies greatly between tissues
- PMID: 23271651
- PMCID: PMC3818247
- DOI: 10.1038/ncomms2325
Activity of the mitochondrial calcium uniporter varies greatly between tissues
Abstract
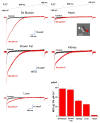
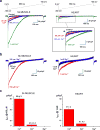
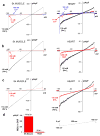
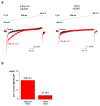
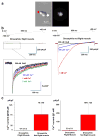
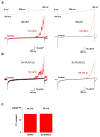
The mitochondrial calcium uniporter is a highly selective channel responsible for mitochondrial Ca(2+) uptake. The mitochondrial calcium uniporter shapes cytosolic Ca(2+) signals, controls mitochondrial ATP production, and is involved in cell death. Here using direct patch-clamp recording from the inner mitochondrial membrane, we compare mitochondrial calcium uniporter activity in mouse heart, skeletal muscle, liver, kidney and brown fat. Surprisingly, heart mitochondria show a dramatically lower mitochondrial calcium uniporter current density than the other tissues studied. Similarly, in Drosophila flight muscle, mitochondrial calcium uniporter activity is barely detectable compared with that in other fly tissues. As mitochondria occupy up to 40% of the cell volume in highly metabolically active heart and flight muscle, low mitochondrial calcium uniporter activity is likely essential to avoid cytosolic Ca(2+) sink due to excessive mitochondrial Ca(2+) uptake. Simultaneously, low mitochondrial calcium uniporter activity may also prevent mitochondrial Ca(2+) overload in such active tissues exposed to frequent cytosolic Ca(2+) activity.
Conflict of interest statement
The authors declare no competing financial interests.
Figures

References
-
- McCormack JG, Halestrap AP, Denton RM. Role of calcium ions in regulation of mammalian intramitochondrial metabolism. Physiol Rev. 1990;70:391–425. - PubMed
-
- Hajnoczky G, Robb-Gaspers LD, Seitz MB, Thomas AP. Decoding of cytosolic calcium oscillations in the mitochondria. Cell. 1995;82:415–424. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

