Extracellular Ca2+ is a danger signal activating the NLRP3 inflammasome through G protein-coupled calcium sensing receptors
- PMID: 23271661
- PMCID: PMC3535422
- DOI: 10.1038/ncomms2339
Extracellular Ca2+ is a danger signal activating the NLRP3 inflammasome through G protein-coupled calcium sensing receptors
Abstract
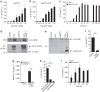
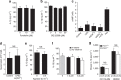
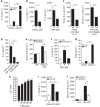
Activation of the NLRP3 inflammasome enables monocytes and macrophages to release high levels of interleukin-1β during inflammatory responses. Concentrations of extracellular calcium can increase at sites of infection, inflammation or cell activation. Here we show that increased extracellular calcium activates the NLRP3 inflammasome via stimulation of G protein-coupled calcium sensing receptors. Activation is mediated by signalling through the calcium-sensing receptor and GPRC6A via the phosphatidyl inositol/Ca(2+) pathway. The resulting increase in the intracellular calcium concentration triggers inflammasome assembly and Caspase-1 activation. We identified necrotic cells as one source for excess extracellular calcium triggering this activation. In vivo, increased calcium concentrations can amplify the inflammatory response in the mouse model of carrageenan-induced footpad swelling, and this effect was inhibited in GPRC6A(-/-) mice. Our results demonstrate that G-protein-coupled receptors can activate the inflammasome, and indicate that increased extracellular calcium has a role as a danger signal and amplifier of inflammation.
Figures


References
-
- Martinon F., Burns K., Tschopp J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol. Cell. 10, 417–426 (2002). - PubMed
-
- Robertson W. G., Marshall R. W. Ionized calcium in body fluids. Crit. Rev. Clin. Lab. Sci. 15, 85–125 (1981). - PubMed
-
- Janle E. M., Sojka J. E. Use of ultrafiltration probes in sheep to collect interstitial fluid for measurement of calcium and magnesium. Contemp. Top. Lab. Anim. Sci. 39, 47–50 (2000). - PubMed
-
- Menkin In Biochemical Mechanisms in Inflammation (ed. Thomas) Springfield, IL (1958).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

