p38α inhibits liver fibrogenesis and consequent hepatocarcinogenesis by curtailing accumulation of reactive oxygen species
- PMID: 23271722
- PMCID: PMC3605785
- DOI: 10.1158/0008-5472.CAN-12-1602
p38α inhibits liver fibrogenesis and consequent hepatocarcinogenesis by curtailing accumulation of reactive oxygen species
Abstract
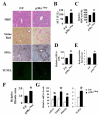
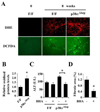
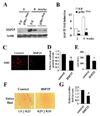
Most hepatocellular carcinomas (HCC) develop in the context of severe liver fibrosis and cirrhosis caused by chronic liver inflammation, which also results in accumulation of reactive oxygen species (ROS). In this study, we examined whether the stress-activated protein kinase p38α (Mapk14) controls ROS metabolism and development of fibrosis and cancer in mice given thioacetamide to induce chronic liver injury. Liver-specific p38α ablation was found to enhance ROS accumulation, which appears to be exerted through the reduced expression of antioxidant protein HSP25 (Hspb1), a mouse homolog of HSP27. Its reexpression in p38α-deficient liver prevents ROS accumulation and thioacetamide-induced fibrosis. p38α deficiency increased expression of SOX2, a marker for cancer stem cells and the liver oncoproteins c-Jun (Jun) and Gankyrin (Psmd10) and led to enhanced thioacetamide-induced hepatocarcinogenesis. The upregulation of SOX2 and c-Jun was prevented by administration of the antioxidant butylated hydroxyanisole. Intriguingly, the risk of human HCC recurrence is positively correlated with ROS accumulation in liver. Thus, p38α and its target HSP25/HSP27 appear to play a conserved and critical hepatoprotective function by curtailing ROS accumulation in liver parenchymal cells engaged in oxidative metabolism of exogenous chemicals. Augmented oxidative stress of liver parenchymal cells may explain the close relationship between liver fibrosis and hepatocarcinogenesis.
Figures


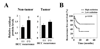
References
-
- Rooney PH, Telfer C, McFadyen MC, Melvin WT, Murray GI. The role of cytochrome P450 in cytotoxic bioactivation: future therapeutic directions. Curr Cancer Drug Targets. 2004;4:257–65. - PubMed
-
- Thorgeirsson SS, Grisham JW. Molecular pathogenesis of human hepatocellular carcinoma. Nat Genet. 2002;31:339–46. - PubMed
-
- Bosch FX, Ribes J, Diaz M, Cleries R. Primary liver cancer: worldwide incidence and trends. Gastroenterology. 2004;127:S5–16. - PubMed
-
- Ginick G. Hepatitis C: controversies, strategies and challenges. Eur J Surg. 1998;S582(Suppl):65–70. - PubMed
-
- Ikeda K, Saitoh S, Suzuki Y, Kobayashi M, Tsubota A, Koida I, et al. Disease progression and hepatocellular carcinogenesis in patients with chronic viral hepatitis: a prospective observation of 2215 patients. J Hepatol. 1998;28:930–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

