The N-terminal 20-amino acid region of guanine nucleotide exchange factor Vav1 plays a distinguished role in T cell receptor-mediated calcium signaling
- PMID: 23271736
- PMCID: PMC3567632
- DOI: 10.1074/jbc.M112.426221
The N-terminal 20-amino acid region of guanine nucleotide exchange factor Vav1 plays a distinguished role in T cell receptor-mediated calcium signaling
Abstract
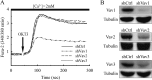
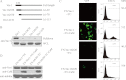
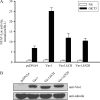
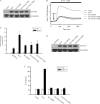
Vav1 is a guanine nucleotide exchange factor (GEF) specifically expressed in hematopoietic cells. It consists of multiple structural domains and plays important roles in T cell activation. The other highly conserved isoforms of Vav family, Vav2 and Vav3, are ubiquitously expressed in human tissues including lymphocytes. All three Vav proteins activate Rho family small GTPases, which are involved in a variety of biological processes during T cell activation. Intensive studies have demonstrated that Vav1 is indispensable for T cell receptor (TCR)-mediated signal transduction, whereas Vav2 and Vav3 function as GEFs that overlap with Vav1 on TCR-induced cytoskeleton reorganization. T cells lacking Vav1 exhibited severe defect in TCR-mediated calcium elevation, indicating that the co-existing Vav2 and Vav3 did not compensate Vav1 in calcium signaling. What is the functional particularity of Vav1 in lymphocytes? In this study, we identified the N-terminal 20 amino acids of Vav1 in the calponin homology (CH) domain to be essential for its interaction with calmodulin (CaM) that leads to TCR-induced calcium mobilization. Substitution of the 1-20 amino acids of Vav1 with those of Vav2 or Vav3 abolished the association with CaM, and the N-terminal mutations of Vav1 failed to potentiate normal TCR-induced calcium mobilization, that in turn, suspended nuclear factor of activated T cells (NFAT) activation and IL-2 production. This study highlights the importance of the N-terminal 20 aa of Vav1 for CaM binding, and provides new insights into the distinguished and irreplaceable role of Vav1 in T cell activation and signal transduction.
Figures



Similar articles
-
The calponin homology domain of Vav1 associates with calmodulin and is prerequisite to T cell antigen receptor-induced calcium release in Jurkat T lymphocytes.J Biol Chem. 2007 Aug 10;282(32):23737-44. doi: 10.1074/jbc.M702975200. Epub 2007 Jun 5. J Biol Chem. 2007. PMID: 17550897
-
Vav family proteins couple to diverse cell surface receptors.Mol Cell Biol. 2000 Sep;20(17):6364-73. doi: 10.1128/MCB.20.17.6364-6373.2000. Mol Cell Biol. 2000. PMID: 10938113 Free PMC article.
-
Vav2 lacks Ca2+ entry-promoting scaffolding functions unique to Vav1 and inhibits T cell activation via Cdc42.J Cell Sci. 2020 Mar 13;133(5):jcs238337. doi: 10.1242/jcs.238337. J Cell Sci. 2020. PMID: 31974114 Free PMC article.
-
Vav-family proteins in T-cell signalling.Curr Opin Immunol. 2005 Jun;17(3):267-74. doi: 10.1016/j.coi.2005.04.003. Curr Opin Immunol. 2005. PMID: 15886116 Review.
-
Vav1: a key signal transducer downstream of the TCR.Immunol Rev. 2003 Apr;192:42-52. doi: 10.1034/j.1600-065x.2003.00032.x. Immunol Rev. 2003. PMID: 12670394 Review.
Cited by
-
Estrogen induces Vav1 expression in human breast cancer cells.PLoS One. 2014 Jun 6;9(6):e99052. doi: 10.1371/journal.pone.0099052. eCollection 2014. PLoS One. 2014. PMID: 24905577 Free PMC article.
-
Succinylation modification-mediated upregulation of Sp1 promotes hepatocellular carcinoma cell proliferation.Discov Oncol. 2024 Nov 15;15(1):660. doi: 10.1007/s12672-024-01533-9. Discov Oncol. 2024. PMID: 39548054 Free PMC article.
-
Phosphatidylinositol Monophosphates Regulate Optimal Vav1 Signaling Output.Cells. 2019 Dec 16;8(12):1649. doi: 10.3390/cells8121649. Cells. 2019. PMID: 31888228 Free PMC article.
-
Reshaping the Immune Microenvironment by Oncolytic Herpes Simplex Virus in Murine Pancreatic Ductal Adenocarcinoma.Mol Ther. 2021 Feb 3;29(2):744-761. doi: 10.1016/j.ymthe.2020.10.027. Epub 2020 Oct 30. Mol Ther. 2021. PMID: 33130310 Free PMC article.
-
The C1 domain of Vav3, a novel potential therapeutic target.Cell Signal. 2017 Dec;40:133-142. doi: 10.1016/j.cellsig.2017.09.008. Epub 2017 Sep 18. Cell Signal. 2017. PMID: 28927664 Free PMC article.
References
-
- Bustelo X. R. (2001) Vav proteins, adaptors and cell signaling. Oncogene 20, 6372–6381 - PubMed
-
- Turner M., Billadeau D. D. (2002) VAV proteins as signal integrators for multi-subunit immune-recognition receptors. Nat. Rev. Immunol. 2, 476–486 - PubMed
-
- Tybulewicz V. L., Ardouin L., Prisco A., Reynolds L. F. (2003) Vav1. A key signal transducer downstream of the TCR. Immunol. Rev. 192, 42–52 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

