Natural killer cells promote immune tolerance by regulating inflammatory TH17 cells at the human maternal-fetal interface
- PMID: 23271808
- PMCID: PMC3549088
- DOI: 10.1073/pnas.1206322110
Natural killer cells promote immune tolerance by regulating inflammatory TH17 cells at the human maternal-fetal interface
Abstract
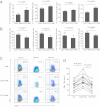
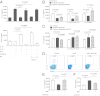
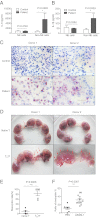
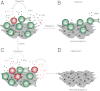
Natural killer (NK) cells accumulate at the maternal-fetal interface in large numbers, but their exact roles in successful pregnancy remain poorly defined. Here, we provide evidence that T(H)17 cells and local inflammation can occur at the maternal-fetal interface during natural allogenic pregnancies. We found that decidual NK cells promote immune tolerance and successful pregnancy by dampening inflammatory T(H)17 cells via IFN-γ secreted by the CD56(bright)CD27(+) NK subset. This NK-cell-mediated regulatory response is lost in patients who experience recurrent spontaneous abortions, which results in a prominent T(H)17 response and extensive local inflammation. This local inflammatory response further affects the regulatory function of NK cells, leading to the eventual loss of maternal-fetal tolerance. Thus, our data identify NK cells as key regulatory cells at the maternal-fetal interface by suppressing T(H)17-mediated local inflammation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
TH1/TH2,3 imbalance due to cytokine-producing NK, gammadelta T and NK-gammadelta T cells in murine pregnancy decidua in success or failure of pregnancy.Am J Reprod Immunol. 2001 May;45(5):257-65. doi: 10.1111/j.8755-8920.2001.450501.x. Am J Reprod Immunol. 2001. PMID: 11432400
-
Mesenchymal stem cells alter the frequency and cytokine profile of natural killer cells in abortion-prone mice.J Cell Physiol. 2020 Oct;235(10):7214-7223. doi: 10.1002/jcp.29620. Epub 2020 Feb 9. J Cell Physiol. 2020. PMID: 32037542
-
Effect of miR-30e regulating NK cell activities on immune tolerance of maternal-fetal interface by targeting PRF1.Biomed Pharmacother. 2019 Jan;109:1478-1487. doi: 10.1016/j.biopha.2018.09.172. Epub 2018 Nov 13. Biomed Pharmacother. 2019. PMID: 30551399
-
Decidual natural killer cells and the immune microenvironment at the maternal-fetal interface.Sci China Life Sci. 2016 Dec;59(12):1224-1231. doi: 10.1007/s11427-016-0337-1. Epub 2016 Nov 29. Sci China Life Sci. 2016. PMID: 27905000 Review.
-
Children's immunology, what can we learn from animal studies (1): Decidual cells induce specific immune system of feto-maternal interface.J Toxicol Sci. 2009;34 Suppl 2:SP331-9. doi: 10.2131/jts.34.sp331. J Toxicol Sci. 2009. PMID: 19571488 Review.
Cited by
-
Tissue-Resident NK Cells: Development, Maturation, and Clinical Relevance.Cancers (Basel). 2020 Jun 12;12(6):1553. doi: 10.3390/cancers12061553. Cancers (Basel). 2020. PMID: 32545516 Free PMC article. Review.
-
The majority of murine γδ T cells at the maternal-fetal interface in pregnancy produce IL-17.Immunol Cell Biol. 2016 Aug;94(7):623-30. doi: 10.1038/icb.2016.48. Epub 2016 May 31. Immunol Cell Biol. 2016. PMID: 27241697
-
Myeloid derived suppressor cells are present at high frequency in neonates and suppress in vitro T cell responses.PLoS One. 2014 Sep 23;9(9):e107816. doi: 10.1371/journal.pone.0107816. eCollection 2014. PLoS One. 2014. PMID: 25248150 Free PMC article.
-
M1/M2 macrophage polarity in normal and complicated pregnancy.Front Immunol. 2014 Nov 24;5:606. doi: 10.3389/fimmu.2014.00606. eCollection 2014. Front Immunol. 2014. PMID: 25505471 Free PMC article. Review.
-
Cellular senescence impact on immune cell fate and function.Aging Cell. 2016 Jun;15(3):400-6. doi: 10.1111/acel.12455. Epub 2016 Feb 22. Aging Cell. 2016. PMID: 26910559 Free PMC article. Review.
References
-
- Sacks GP, Studena K, Sargent K, Redman CW. Normal pregnancy and preeclampsia both produce inflammatory changes in peripheral blood leukocytes akin to those of sepsis. Am J Obstet Gynecol. 1998;179(1):80–86. - PubMed
-
- Trowsdale J, Betz AG. Mother’s little helpers: Mechanisms of maternal-fetal tolerance. Nat Immunol. 2006;7(3):241–246. - PubMed
-
- Stillerman KP, Mattison DR, Giudice LC, Woodruff TJ. 2008. Environmental exposures and adverse pregnancy outcomes: A review of the science. Reprod Sci 15(7):631–650. - PubMed
-
- Sargent IL, Borzychowski AM, Redman CW. NK cells and human pregnancy—An inflammatory view. Trends Immunol. 2006;27(9):399–404. - PubMed
-
- Karimi K, Arck PC. Keepers of pregnancy in the turnstile of the environment. Brain Behav Immun. 2010;24(3):339–347. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

