Liquid transport facilitated by channels in Bacillus subtilis biofilms
- PMID: 23271809
- PMCID: PMC3549102
- DOI: 10.1073/pnas.1216376110
Liquid transport facilitated by channels in Bacillus subtilis biofilms
Abstract
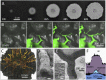
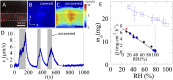
Many bacteria on earth exist in surface-attached communities known as biofilms. These films are responsible for manifold problems, including hospital-acquired infections and biofouling, but they can also be beneficial. Biofilm growth depends on the transport of nutrients and waste, for which diffusion is thought to be the main source of transport. However, diffusion is ineffective for transport over large distances and thus should limit growth. Nevertheless, biofilms can grow to be very large. Here we report the presence of a remarkable network of well-defined channels that form in wild-type Bacillus subtilis biofilms and provide a system for enhanced transport. We observe that these channels have high permeability to liquid flow and facilitate the transport of liquid through the biofilm. In addition, we find that spatial variations in evaporative flux from the surface of these biofilms provide a driving force for the flow of liquid in the channels. These channels offer a remarkably simple system for liquid transport, and their discovery provides insight into the physiology and growth of biofilms.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Flemming H-C, Wingender J. The biofilm matrix. Nat Rev Microbiol. 2010;8(9):623–633. - PubMed
-
- Sutherland IW. Biofilm exopolysaccharides: A strong and sticky framework. Microbiology. 2001;147(Pt 1):3–9. - PubMed
-
- Branda SS, Vik S, Friedman L, Kolter R. Biofilms: The matrix revisited. Trends Microbiol. 2005;13(1):20–26. - PubMed
-
- Ghannoum M, O’Toole GA, editors. Microbial Biofilms. Washington, DC: ASM Press; 2004.
-
- O’Toole G, Kaplan HB, Kolter R. Biofilm formation as microbial development. Annu Rev Microbiol. 2000;54:49–79. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

