Siglec-1 is a novel dendritic cell receptor that mediates HIV-1 trans-infection through recognition of viral membrane gangliosides
- PMID: 23271952
- PMCID: PMC3525531
- DOI: 10.1371/journal.pbio.1001448
Siglec-1 is a novel dendritic cell receptor that mediates HIV-1 trans-infection through recognition of viral membrane gangliosides
Abstract
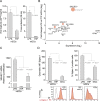
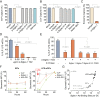
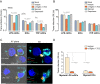
Dendritic cells (DCs) are essential antigen-presenting cells for the induction of immunity against pathogens. However, HIV-1 spread is strongly enhanced in clusters of DCs and CD4(+) T cells. Uninfected DCs capture HIV-1 and mediate viral transfer to bystander CD4(+) T cells through a process termed trans-infection. Initial studies identified the C-type lectin DC-SIGN as the HIV-1 binding factor on DCs, which interacts with the viral envelope glycoproteins. Upon DC maturation, however, DC-SIGN is down-regulated, while HIV-1 capture and trans-infection is strongly enhanced via a glycoprotein-independent capture pathway that recognizes sialyllactose-containing membrane gangliosides. Here we show that the sialic acid-binding Ig-like lectin 1 (Siglec-1, CD169), which is highly expressed on mature DCs, specifically binds HIV-1 and vesicles carrying sialyllactose. Furthermore, Siglec-1 is essential for trans-infection by mature DCs. These findings identify Siglec-1 as a key factor for HIV-1 spread via infectious DC/T-cell synapses, highlighting a novel mechanism that mediates HIV-1 dissemination in activated tissues.
Conflict of interest statement
I have read the journal's policy and have the following conflicts: A patent application based on this work has been filed (EP11382392.6, 2011). The authors declare that no other competing financial interests exist.
Figures


Comment in
-
How HIV sneaks aboard mature dendritic cells.PLoS Biol. 2012;10(12):e1001454. doi: 10.1371/journal.pbio.1001454. Epub 2012 Dec 18. PLoS Biol. 2012. PMID: 23271956 Free PMC article. No abstract available.
References
-
- Cameron PU, Freudenthal PS, Barker JM, Gezelter S, Inaba K, Steinman RM (1992) Dendritic cells exposed to human immunodeficiency virus type-1 transmit a vigorous cytopathic infection to CD4+ T cells. Science 257: 383–387. - PubMed
-
- Geijtenbeek TB, Kwon DS, Torensma R, van Vliet SJ, van Duijnhoven GC, et al. (2000) DC-SIGN, a dendritic cell-specific HIV-1-binding protein that enhances trans-infection of T cells. Cell 100: 587–597. - PubMed
-
- McDonald D, Wu L, Bohks SM, KewalRamani VN, Unutmaz D, Hope TJ (2003) Recruitment of HIV and its receptors to dendritic cell-T cell junctions. Science 300: 1295–1297. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

