Climbing fiber burst size and olivary sub-threshold oscillations in a network setting
- PMID: 23271962
- PMCID: PMC3521668
- DOI: 10.1371/journal.pcbi.1002814
Climbing fiber burst size and olivary sub-threshold oscillations in a network setting
Abstract
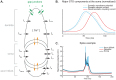
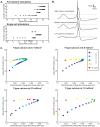
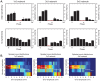
The inferior olivary nucleus provides one of the two main inputs to the cerebellum: the so-called climbing fibers. Activation of climbing fibers is generally believed to be related to timing of motor commands and/or motor learning. Climbing fiber spikes lead to large all-or-none action potentials in cerebellar Purkinje cells, overriding any other ongoing activity and silencing these cells for a brief period of time afterwards. Empirical evidence shows that the climbing fiber can transmit a short burst of spikes as a result of an olivary cell somatic spike, potentially increasing the information being transferred to the cerebellum per climbing fiber activation. Previously reported results from in vitro studies suggested that the information encoded in the climbing fiber burst is related to the occurrence of the spike relative to the ongoing sub-threshold membrane potential oscillation of the olivary cell, i.e. that the phase of the oscillation is reflected in the size of the climbing fiber burst. We used a detailed three-compartmental model of an inferior olivary cell to further investigate the possible factors determining the size of the climbing fiber burst. Our findings suggest that the phase-dependency of the burst size is present but limited and that charge flow between soma and dendrite is a major determinant of the climbing fiber burst. From our findings it follows that phenomena such as cell ensemble synchrony can have a big effect on the climbing fiber burst size through dendrodendritic gap-junctional coupling between olivary cells.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

Similar articles
-
Association between dendritic lamellar bodies and complex spike synchrony in the olivocerebellar system.J Neurophysiol. 1997 Apr;77(4):1747-58. doi: 10.1152/jn.1997.77.4.1747. J Neurophysiol. 1997. PMID: 9114233
-
Olivary subthreshold oscillations and burst activity revisited.Front Neural Circuits. 2012 Nov 22;6:91. doi: 10.3389/fncir.2012.00091. eCollection 2012. Front Neural Circuits. 2012. PMID: 23189043 Free PMC article.
-
Reading the clock: how Purkinje cells decode the phase of olivary oscillations.Neuron. 2009 May 14;62(3):308-9. doi: 10.1016/j.neuron.2009.04.020. Neuron. 2009. PMID: 19447086
-
Complex Spike Wars: a New Hope.Cerebellum. 2018 Dec;17(6):735-746. doi: 10.1007/s12311-018-0960-3. Cerebellum. 2018. PMID: 29982917 Free PMC article. Review.
-
Inhibitory control of olivary discharge.Ann N Y Acad Sci. 2002 Dec;978:219-31. doi: 10.1111/j.1749-6632.2002.tb07569.x. Ann N Y Acad Sci. 2002. PMID: 12582055 Review.
Cited by
-
Heterogeneous Expression of T-type Ca(2+) Channels Defines Different Neuronal Populations in the Inferior Olive of the Mouse.Front Cell Neurosci. 2016 Aug 4;10:192. doi: 10.3389/fncel.2016.00192. eCollection 2016. Front Cell Neurosci. 2016. PMID: 27540355 Free PMC article.
-
Electrical coupling controls dimensionality and chaotic firing of inferior olive neurons.PLoS Comput Biol. 2020 Jul 30;16(7):e1008075. doi: 10.1371/journal.pcbi.1008075. eCollection 2020 Jul. PLoS Comput Biol. 2020. PMID: 32730255 Free PMC article.
-
Duration of Purkinje cell complex spikes increases with their firing frequency.Front Cell Neurosci. 2015 Apr 13;9:122. doi: 10.3389/fncel.2015.00122. eCollection 2015. Front Cell Neurosci. 2015. PMID: 25918500 Free PMC article.
-
Modeling the Cerebellar Microcircuit: New Strategies for a Long-Standing Issue.Front Cell Neurosci. 2016 Jul 8;10:176. doi: 10.3389/fncel.2016.00176. eCollection 2016. Front Cell Neurosci. 2016. PMID: 27458345 Free PMC article. Review.
-
Conversion of Graded Presynaptic Climbing Fiber Activity into Graded Postsynaptic Ca2+ Signals by Purkinje Cell Dendrites.Neuron. 2019 May 22;102(4):762-769.e4. doi: 10.1016/j.neuron.2019.03.010. Epub 2019 Mar 27. Neuron. 2019. PMID: 30928170 Free PMC article.
References
-
- De Zeeuw CI, Hoebeek FE, Bosman LW, Schonewille M, Witter L, et al. (2011) Spatiotemporal firing patterns in the cerebellum. Nat Rev Neurosci 12(6): 327–44. - PubMed
-
- Eccles J, Llinás R, Sasaki K (1964) Excitation of cerebellar Purkinje cells by the climbing fibres. Nature 203: 245–246. - PubMed
-
- Deuschl G, Toro C, Valls-Solé J, Zeffiro T, Zee DS, et al. (1994) Symptomatic and essential palatal tremor. 1. Clinical, physiological and MRI analysis. Brain 117: 775–788. - PubMed
-
- Holmberg M, Duyckaerts C, Dürr A, Cancel G, Gourfinkel-An I, et al. (1998) Spinocerebellar ataxia type 7 (SCA7): a neurodegenerative disorder with neuronal intranuclear inclusions. Hum Mol Genet 7: 913–918. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

