A mechanism of gene amplification driven by small DNA fragments
- PMID: 23271978
- PMCID: PMC3521702
- DOI: 10.1371/journal.pgen.1003119
A mechanism of gene amplification driven by small DNA fragments
Abstract
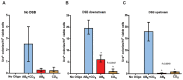
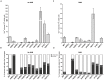
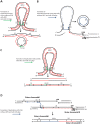
DNA amplification is a molecular process that increases the copy number of a chromosomal tract and often causes elevated expression of the amplified gene(s). Although gene amplification is frequently observed in cancer and other degenerative disorders, the molecular mechanisms involved in the process of DNA copy number increase remain largely unknown. We hypothesized that small DNA fragments could be the trigger of DNA amplification events. Following our findings that small fragments of DNA in the form of DNA oligonucleotides can be highly recombinogenic, we have developed a system in the yeast Saccharomyces cerevisiae to capture events of chromosomal DNA amplification initiated by small DNA fragments. Here we demonstrate that small DNAs can amplify a chromosomal region, generating either tandem duplications or acentric extrachromosomal DNA circles. Small fragment-driven DNA amplification (SFDA) occurs with a frequency that increases with the length of homology between the small DNAs and the target chromosomal regions. SFDA events are triggered even by small single-stranded molecules with as little as 20-nt homology with the genomic target. A double-strand break (DSB) external to the chromosomal amplicon region stimulates the amplification event up to a factor of 20 and favors formation of extrachromosomal circles. SFDA is dependent on Rad52 and Rad59, partially dependent on Rad1, Rad10, and Pol32, and independent of Rad51, suggesting a single-strand annealing mechanism. Our results reveal a novel molecular model for gene amplification, in which small DNA fragments drive DNA amplification and define the boundaries of the amplicon region. As DNA fragments are frequently found both inside cells and in the extracellular environment, such as the serum of patients with cancer or other degenerative disorders, we propose that SFDA may be a common mechanism for DNA amplification in cancer cells, as well as a more general cause of DNA copy number variation in nature.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



Similar articles
-
Rad51 protein controls Rad52-mediated DNA annealing.J Biol Chem. 2008 May 23;283(21):14883-92. doi: 10.1074/jbc.M801097200. Epub 2008 Mar 12. J Biol Chem. 2008. PMID: 18337252 Free PMC article.
-
The Rad52-Rad59 complex interacts with Rad51 and replication protein A.DNA Repair (Amst). 2003 Oct 7;2(10):1127-34. doi: 10.1016/s1568-7864(03)00121-6. DNA Repair (Amst). 2003. PMID: 13679150
-
Rad52 and Rad59 exhibit both overlapping and distinct functions.DNA Repair (Amst). 2007 Jan 4;6(1):27-37. doi: 10.1016/j.dnarep.2006.08.007. Epub 2006 Sep 20. DNA Repair (Amst). 2007. PMID: 16987715
-
Emerging non-canonical roles for the Rad51-Rad52 interaction in response to double-strand breaks in yeast.Curr Genet. 2020 Oct;66(5):917-926. doi: 10.1007/s00294-020-01081-z. Epub 2020 May 12. Curr Genet. 2020. PMID: 32399607 Free PMC article. Review.
-
Gene amplifications and extrachromosomal circular DNAs: function and biogenesis.Mol Biol Rep. 2023 Sep;50(9):7693-7703. doi: 10.1007/s11033-023-08649-1. Epub 2023 Jul 11. Mol Biol Rep. 2023. PMID: 37433908 Review.
Cited by
-
The EccDNA Replicon: A Heritable, Extranuclear Vehicle That Enables Gene Amplification and Glyphosate Resistance in Amaranthus palmeri.Plant Cell. 2020 Jul;32(7):2132-2140. doi: 10.1105/tpc.20.00099. Epub 2020 Apr 23. Plant Cell. 2020. PMID: 32327538 Free PMC article.
-
Extrachromosomal circular DNA is common in yeast.Proc Natl Acad Sci U S A. 2015 Jun 16;112(24):E3114-22. doi: 10.1073/pnas.1508825112. Epub 2015 Jun 2. Proc Natl Acad Sci U S A. 2015. PMID: 26038577 Free PMC article.
-
The role of extracellular DNA (exDNA) in cellular processes.Cancer Biol Ther. 2021 Apr 3;22(4):267-278. doi: 10.1080/15384047.2021.1890319. Epub 2021 Apr 16. Cancer Biol Ther. 2021. PMID: 33858306 Free PMC article. Review.
-
HER2 Overexpression and Cytogenetical Patterns in Canine Mammary Carcinomas.Vet Sci. 2022 Oct 22;9(11):583. doi: 10.3390/vetsci9110583. Vet Sci. 2022. PMID: 36356060 Free PMC article.
-
Palindromic amplification of the ERBB2 oncogene in primary HER2-positive breast tumors.Sci Rep. 2017 Feb 17;7:41921. doi: 10.1038/srep41921. Sci Rep. 2017. PMID: 28211519 Free PMC article.
References
-
- Albertson DG (2006) Gene amplification in cancer. Trends Genet 22: 447–455. - PubMed
-
- Albertson DG, Collins C, McCormick F, Gray JW (2003) Chromosome aberrations in solid tumors. Nat Genet 34: 369–376. - PubMed
-
- Schimke RT (1984) Gene amplification, drug resistance, and cancer. Cancer Res 44: 1735–1742. - PubMed
-
- Hattinger CM, Stoico G, Michelacci F, Pasello M, Scionti I, et al. (2009) Mechanisms of gene amplification and evidence of coamplification in drug-resistant human osteosarcoma cell lines. Genes Chromosomes Cancer 48: 289–309. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

