Listeria monocytogenes infection causes metabolic shifts in Drosophila melanogaster
- PMID: 23272066
- PMCID: PMC3521769
- DOI: 10.1371/journal.pone.0050679
Listeria monocytogenes infection causes metabolic shifts in Drosophila melanogaster
Abstract
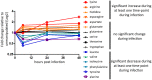
Immunity and metabolism are intimately linked; manipulating metabolism, either through diet or genetics, has the power to alter survival during infection. However, despite metabolism's powerful ability to alter the course of infections, little is known about what being "sick" means metabolically. Here we describe the metabolic changes occurring in a model system when Listeria monocytogenes causes a lethal infection in Drosophila melanogaster. L. monocytogenes infection alters energy metabolism; the flies gradually lose both of their energy stores, triglycerides and glycogen, and show decreases in both intermediate metabolites and enzyme message for the two main energy pathways, beta-oxidation and glycolysis. L. monocytogenes infection also causes enzymatic reduction in the anti-oxidant uric acid, and knocking out the enzyme uric oxidase has a complicated effect on immunity. Free amino acid levels also change during infection, including a drop in tyrosine levels which may be due to robust L. monocytogenes induced melanization.
Conflict of interest statement
Figures





References
-
- Dionne MS, Pham LN, Shirasu-Hiza M, Schneider DS (2006) Akt and FOXO dysregulation contribute to infection-induced wasting in Drosophila. Curr Biol 16: 1977–1985. - PubMed
-
- Becker T, Loch G, Beyer M, Zinke I, Aschenbrenner AC, et al. (2010) FOXO-dependent regulation of innate immune homeostasis. Nature 463: 369–373. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

