δ-opioid receptor activation and microRNA expression of the rat cortex in hypoxia
- PMID: 23272113
- PMCID: PMC3521741
- DOI: 10.1371/journal.pone.0051524
δ-opioid receptor activation and microRNA expression of the rat cortex in hypoxia
Abstract
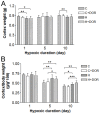
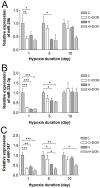
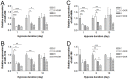
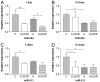
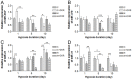
Prolonged hypoxic/ischemic stress may cause cortical injury and clinically manifest as a neurological disability. Activation of the δ-opioid receptor (DOR) may induce cortical protection against hypoxic/ischemic insults. However, the mechanisms underlying DOR protection are not clearly understood. We have recently found that DOR activation modulates the expression of microRNAs (miRNAs) in the kidney exposed to hypoxia, suggesting that DOR protection may involve a miRNA mechanism. To determine if the miRNAs expressed in the cortex mediated DOR neuroprotection, we examined 19 miRNAs that were previously identified as hypoxia- and DOR-regulated miRNAs in the kidney, in the rat cortex treated with UFP-512, a potent and specific DOR agonist under hypoxic condition. Of the 19 miRNAs tested, 17 were significantly altered by hypoxia and/or DOR activation with the direction and amplitude varying depending on hypoxic duration and times of DOR treatment. Expression of several miRNAs such as miR-29b, -101b, -298, 324-3p, -347 and 466b was significantly depressed after 24 hours of hypoxia. Similar changes were seen in normoxic condition 24 hours after DOR activation with one-time treatment of UFP-512. In contrast, some miRNAs were more tolerant to hypoxic stress and showed significant reduction only with 5-day (e.g., miR-31 and -186) or 10-day (e.g., miR-29a, let-7f and -511) exposures. In addition, these miRNAs had differential responses to DOR activation. Other miRNAs like miRs-363* and -370 responded only to the combined exposure to hypoxia and DOR treatment, with a notable reduction of >70% in the 5-day group. These data suggest that cortical miRNAs are highly yet differentially sensitive to hypoxia. DOR activation can modify, enhance or resolve the changes in miRNAs that target HIF, ion transport, axonal guidance, free radical signaling, apoptosis and many other functions.
Conflict of interest statement
Figures

References
-
- Zhang J, Haddad GG, Xia Y (2000) delta-, but not mu- and kappa-, opioid receptor activation protects neocortical neurons from glutamate-induced excitotoxic injury. Brain Res 885: 143–153. - PubMed
-
- Zhang J, Gibney GT, Zhao P, Xia Y (2002) Neuroprotective role of delta-opioid receptors in cortical neurons. Am J Physiol Cell Physiol 282: C1225–1234. - PubMed
-
- Ma MC, Qian H, Ghassemi F, Zhao P, Xia Y (2005) Oxygen-sensitive {delta}-opioid receptor-regulated survival and death signals: novel insights into neuronal preconditioning and protection. J Biol Chem 280: 16208–16218. - PubMed
-
- Zhang J, Qian H, Zhao P, Hong SS, Xia Y (2006) Rapid hypoxia preconditioning protects cortical neurons from glutamate toxicity through delta-opioid receptor. Stroke 37: 1094–1099. - PubMed
-
- Chao D, Donnelly DF, Feng Y, Bazzy-Asaad A, Xia Y (2007) Cortical delta-opioid receptors potentiate K+ homeostasis during anoxia and oxygen-glucose deprivation. J Cereb Blood Flow Metab 27: 356–368. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

