Prophylactic valproic acid treatment prevents schizophrenia-related behaviour in Disc1-L100P mutant mice
- PMID: 23272119
- PMCID: PMC3525594
- DOI: 10.1371/journal.pone.0051562
Prophylactic valproic acid treatment prevents schizophrenia-related behaviour in Disc1-L100P mutant mice
Abstract
Background: Schizophrenia is a neurodevelopmental disorder with onset early in adulthood. Disrupted-In-Schizophrenia-1 (DISC1) is a susceptibility gene for schizophrenia and other psychiatric disorders. Disc1-L100P mutant mice show behaviors relevant to schizophrenia at 12 weeks, but not at 8 weeks of age, and may be useful for investigating the onset of schizophrenia in early adulthood.
Methods: We investigated whether early valproic acid treatment would prevent behavioral, cellular and gene expression abnormalities in Disc1-L100P mutants.
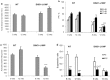
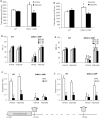
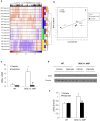
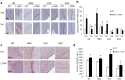
Results: Valproic acid prevented hyperactivity and deficits in prepulse inhibition and latent inhibition in Disc1-L100P mice. Genome-wide transcription profiling identified Lcn2 (lipocalin2) transcripts as being elevated by the Disc1 mutation and corrected by valproate. Disc1-L100P mice also had increased glial cell numbers in the subventricular zone, which was normalized by valproate. Genetic deletion of Lcn2 normalized glial cell numbers and behavior in Disc1-L100P mutants.
Conclusions: Pharmacological treatments are a feasible way of preventing abnormal behaviour in a genetic model of schizophrenia. Lcn2 is a potential novel drug target for early intervention in schizophrenia.
Conflict of interest statement
Figures

References
-
- Lewis DA, Levitt P (2002) Schizophrenia as a disorder of neurodevelopment. Annu Rev Neurosci 25: 409–432. Epub 2002 Mar 2022. - PubMed
-
- Enomoto A, Asai N, Namba T, Wang Y, Kato T, et al. (2009) Roles of disrupted-in-schizophrenia 1-interacting protein girdin in postnatal development of the dentate gyrus. Neuron 63: 774–787. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

