The role of human Dicer-dsRBD in processing small regulatory RNAs
- PMID: 23272173
- PMCID: PMC3521659
- DOI: 10.1371/journal.pone.0051829
The role of human Dicer-dsRBD in processing small regulatory RNAs
Abstract
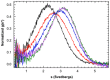
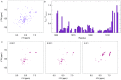
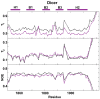
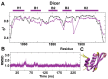
One of the most exciting recent developments in RNA biology has been the discovery of small non-coding RNAs that affect gene expression through the RNA interference (RNAi) mechanism. Two major classes of RNAs involved in RNAi are small interfering RNA (siRNA) and microRNA (miRNA). Dicer, an RNase III enzyme, plays a central role in the RNAi pathway by cleaving precursors of both of these classes of RNAs to form mature siRNAs and miRNAs, which are then loaded into the RNA-induced silencing complex (RISC). miRNA and siRNA precursors are quite structurally distinct; miRNA precursors are short, imperfect hairpins while siRNA precursors are long, perfect duplexes. Nonetheless, Dicer is able to process both. Dicer, like the majority of RNase III enzymes, contains a dsRNA binding domain (dsRBD), but the data are sparse on the exact role this domain plays in the mechanism of Dicer binding and cleavage. To further explore the role of human Dicer-dsRBD in the RNAi pathway, we determined its binding affinity to various RNAs modeling both miRNA and siRNA precursors. Our study shows that Dicer-dsRBD is an avid binder of dsRNA, but its binding is only minimally influenced by a single-stranded - double-stranded junction caused by large terminal loops observed in miRNA precursors. Thus, the Dicer-dsRBD contributes directly to substrate binding but not to the mechanism of differentiating between pre-miRNA and pre-siRNA. In addition, NMR spin relaxation and MD simulations provide an overview of the role that dynamics contribute to the binding mechanism. We compare this current study with our previous studies of the dsRBDs from Drosha and DGCR8 to give a dynamic profile of dsRBDs in their apo-state and a mechanistic view of dsRNA binding by dsRBDs in general.
Conflict of interest statement
Figures



References
-
- Kim VN, Han J, Siomi MC (2009) Biogenesis of small RNAs in animals. Nat Rev Mol Cell Biol 10: 126–139. - PubMed
-
- Jinek M, Doudna JA (2009) A three-dimensional view of the molecular machinery of RNA interference. Nature 457: 405–412. - PubMed
-
- van Rij RP, Berezikov E (2009) Small RNAs and the control of transposons and viruses in Drosophila. Trends Microbiol 17: 163–171. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

