Evaluation of biological properties of electron beam melted Ti6Al4V implant with biomimetic coating in vitro and in vivo
- PMID: 23272208
- PMCID: PMC3525565
- DOI: 10.1371/journal.pone.0052049
Evaluation of biological properties of electron beam melted Ti6Al4V implant with biomimetic coating in vitro and in vivo
Abstract
Background: High strength porous titanium implants are widely used for the reconstruction of craniofacial defects because of their similar mechanical properties to those of bone. The recent introduction of electron beam melting (EBM) technique allows a direct digitally enabled fabrication of patient specific porous titanium implants, whereas both their in vitro and in vivo biological performance need further investigation.
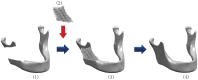
Methods: In the present study, we fabricated porous Ti6Al4V implants with controlled porous structure by EBM process, analyzed their mechanical properties, and conducted the surface modification with biomimetic approach. The bioactivities of EBM porous titanium in vitro and in vivo were evaluated between implants with and without biomimetic apatite coating.
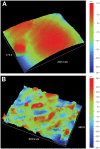
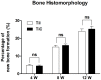
Results: The physical property of the porous implants, containing the compressive strength being 163 - 286 MPa and the Young's modulus being 14.5-38.5 GPa, is similar to cortical bone. The in vitro culture of osteoblasts on the porous Ti6Al4V implants has shown a favorable circumstance for cell attachment and proliferation as well as cell morphology and spreading, which were comparable with the implants coating with bone-like apatite. In vivo, histological analysis has obtained a rapid ingrowth of bone tissue from calvarial margins toward the center of bone defect in 12 weeks. We observed similar increasing rate of bone ingrowth and percentage of bone formation within coated and uncoated implants, all of which achieved a successful bridging of the defect in 12 weeks after the implantation.
Conclusions: This study demonstrated that the EBM porous Ti6Al4V implant not only reduced the stress-shielding but also exerted appropriate osteoconductive properties, as well as the apatite coated group. The results opened up the possibility of using purely porous titanium alloy scaffolds to reconstruct specific bone defects in the maxillofacial and orthopedic fields.
Conflict of interest statement
Figures







Similar articles
-
Mechanical evaluation of porous titanium (Ti6Al4V) structures with electron beam melting (EBM).J Mech Behav Biomed Mater. 2010 Apr;3(3):249-59. doi: 10.1016/j.jmbbm.2009.10.006. Epub 2009 Oct 22. J Mech Behav Biomed Mater. 2010. PMID: 20142109
-
Bionic mechanical design and 3D printing of novel porous Ti6Al4V implants for biomedical applications.J Zhejiang Univ Sci B. 2019 Aug.;20(8):647-659. doi: 10.1631/jzus.B1800622. J Zhejiang Univ Sci B. 2019. PMID: 31273962 Free PMC article.
-
Microstructure and mechanical properties of porous titanium structures fabricated by electron beam melting for cranial implants.Proc Inst Mech Eng H. 2018 Feb;232(2):185-199. doi: 10.1177/0954411917751558. Epub 2018 Jan 13. Proc Inst Mech Eng H. 2018. Retraction in: Proc Inst Mech Eng H. 2018 Dec;232(12):1271. doi: 10.1177/0954411918804609. PMID: 29332500 Retracted.
-
Complex geometry and integrated macro-porosity: Clinical applications of electron beam melting to fabricate bespoke bone-anchored implants.Acta Biomater. 2023 Jan 15;156:125-145. doi: 10.1016/j.actbio.2022.06.002. Epub 2022 Jun 5. Acta Biomater. 2023. PMID: 35675890 Review.
-
A systematic review of preclinical in vivo testing of 3D printed porous Ti6Al4V for orthopedic applications, part I: Animal models and bone ingrowth outcome measures.J Biomed Mater Res B Appl Biomater. 2021 Oct;109(10):1436-1454. doi: 10.1002/jbm.b.34803. Epub 2021 Jan 22. J Biomed Mater Res B Appl Biomater. 2021. PMID: 33484102
Cited by
-
Improving osteoinduction and osteogenesis of Ti6Al4V alloy porous scaffold by regulating the pore structure.Front Chem. 2023 May 17;11:1190630. doi: 10.3389/fchem.2023.1190630. eCollection 2023. Front Chem. 2023. PMID: 37265590 Free PMC article.
-
Mechanical behavior of a titanium alloy scaffold mimicking trabecular structure.J Orthop Surg Res. 2020 Feb 7;15(1):40. doi: 10.1186/s13018-019-1489-y. J Orthop Surg Res. 2020. PMID: 32028970 Free PMC article.
-
The Recent Revolution in the Design and Manufacture of Cranial Implants: Modern Advancements and Future Directions.Neurosurgery. 2015 Nov;77(5):814-24; discussion 824. doi: 10.1227/NEU.0000000000000899. Neurosurgery. 2015. PMID: 26171578 Free PMC article. Review.
-
Potential use of porous titanium-niobium alloy in orthopedic implants: preparation and experimental study of its biocompatibility in vitro.PLoS One. 2013 Nov 19;8(11):e79289. doi: 10.1371/journal.pone.0079289. eCollection 2013. PLoS One. 2013. PMID: 24260188 Free PMC article.
-
Osseointegration in additive-manufactured titanium implants: A systematic review of animal studies on the need for surface treatment.Heliyon. 2023 Jun 10;9(6):e17105. doi: 10.1016/j.heliyon.2023.e17105. eCollection 2023 Jun. Heliyon. 2023. PMID: 37484223 Free PMC article.
References
-
- Kroger H, Venesmaa P, Jurvelin J, Miettinen H, Suomalainen O, et al... (1998) Bone density at the proximal femur after total hip arthroplasty. Clin Orthop Relat Res: 66–74. - PubMed
-
- Ryan G, Pandit A, Apatsidis DP (2006) Fabrication methods of porous metals for use in orthopaedic applications. Biomaterials 27: 2651–2670. - PubMed
-
- Kienapfel H, Sprey C, Wilke A, Griss P (1999) Implant fixation by bone ingrowth. J Arthroplasty 14: 355–368. - PubMed
-
- Li J, Habibovic P, Yuan H, van den Doel M, Wilson CE, et al. (2007) Biological performance in goats of a porous titanium alloy-biphasic calcium phosphate composite. Biomaterials 28: 4209–4218. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

