25-hydroxyvitamin D₃ and 1,25-dihydroxyvitamin D₃ promote the differentiation of human subcutaneous preadipocytes
- PMID: 23272223
- PMCID: PMC3525569
- DOI: 10.1371/journal.pone.0052171
25-hydroxyvitamin D₃ and 1,25-dihydroxyvitamin D₃ promote the differentiation of human subcutaneous preadipocytes
Abstract
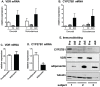
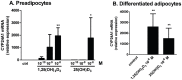
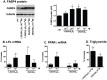
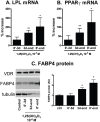
1,25(OH)(2)D(3) inhibits adipogenesis in mouse 3T3-L1 adipocytes, but little is known about its effects or local metabolism in human adipose tissue. We showed that vitamin D receptor (VDR) and 1α-hydroxylase (CYP27B1), the enzyme that activates 25(OH)D(3) to 1,25(OH)(2)D(3), were expressed in human adipose tissues, primary preadipocytes and newly-differentiated adipocytes. Preadipocytes and newly-differentiated adipocytes were responsive to 1,25(OH)(2)D(3), as indicated by a markedly increased expression of CYP24A1, a primary VDR target. 1,25(OH)(2)D(3) enhanced adipogenesis as determined by increased expression of adipogenic markers and triglyceride accumulation (50% to 150%). The magnitude of the effect was greater in the absence of thiazolidinediones. 1,25(OH)(2)D(3) was equally effective when added after the removal of differentiation cocktail on day 3, but it had no effect when added only during the induction period (day 0-3), suggesting that 1,25(OH)(2)D(3) promoted maturation. 25(OH)D(3) also stimulated CYP24A1 expression and adipogenesis, most likely through its conversion to 1,25(OH)(2)D(3). Consistent with this possibility, incubation of preadipocytes with 25(OH)D(3) led to 1,25(OH)(2)D(3) accumulation in the media. 1,25(OH)(2)D(3) also enhanced adipogenesis in primary mouse preadipocytes. We conclude that vitamin D status may regulate human adipose tissue growth and remodeling.
Conflict of interest statement
Figures


Similar articles
-
Leptin blocks the inhibitory effect of vitamin D on adipogenesis and cell proliferation in 3T3-L1 adipocytes.Gen Comp Endocrinol. 2018 Sep 15;266:1-8. doi: 10.1016/j.ygcen.2018.01.014. Epub 2018 Jan 12. Gen Comp Endocrinol. 2018. PMID: 29339180
-
Altered pharmacokinetics of 1alpha,25-dihydroxyvitamin D3 and 25-hydroxyvitamin D3 in the blood and tissues of the 25-hydroxyvitamin D-24-hydroxylase (Cyp24a1) null mouse.Endocrinology. 2005 Feb;146(2):825-34. doi: 10.1210/en.2004-1116. Epub 2004 Oct 21. Endocrinology. 2005. PMID: 15498883
-
Molecular mechanism of 1,25-dihydroxyvitamin D3 inhibition of adipogenesis in 3T3-L1 cells.Am J Physiol Endocrinol Metab. 2006 May;290(5):E916-24. doi: 10.1152/ajpendo.00410.2005. Epub 2005 Dec 20. Am J Physiol Endocrinol Metab. 2006. PMID: 16368784
-
Analysis of vitamin D metabolism gene expression in human bone: evidence for autocrine control of bone remodelling.J Steroid Biochem Mol Biol. 2014 Oct;144 Pt A:110-3. doi: 10.1016/j.jsbmb.2013.09.016. Epub 2013 Oct 10. J Steroid Biochem Mol Biol. 2014. PMID: 24120913 Review.
-
The role of Vitamin D3 metabolism in prostate cancer.J Steroid Biochem Mol Biol. 2004 Nov;92(4):317-25. doi: 10.1016/j.jsbmb.2004.10.007. Epub 2004 Dec 19. J Steroid Biochem Mol Biol. 2004. PMID: 15663995 Review.
Cited by
-
Vitamin D Receptor Gene Expression in Adipose Tissue of Obese Individuals is Regulated by miRNA and Correlates with the Pro-Inflammatory Cytokine Level.Int J Mol Sci. 2019 Oct 24;20(21):5272. doi: 10.3390/ijms20215272. Int J Mol Sci. 2019. PMID: 31652924 Free PMC article.
-
Mobilising vitamin D from adipose tissue: The potential impact of exercise.Nutr Bull. 2019 Mar;44(1):25-35. doi: 10.1111/nbu.12369. Epub 2019 Feb 3. Nutr Bull. 2019. PMID: 34853551 Free PMC article. Review.
-
Evaluation of association studies and a systematic review and meta-analysis of VDR polymorphisms in type 2 diabetes mellitus risk.Medicine (Baltimore). 2021 Jul 16;100(28):e25934. doi: 10.1097/MD.0000000000025934. Medicine (Baltimore). 2021. PMID: 34260520 Free PMC article.
-
The Molecular Mechanisms by Which Vitamin D Prevents Insulin Resistance and Associated Disorders.Int J Mol Sci. 2020 Sep 11;21(18):6644. doi: 10.3390/ijms21186644. Int J Mol Sci. 2020. PMID: 32932777 Free PMC article. Review.
-
Vitamin D Status, Calcium Intake and Risk of Developing Type 2 Diabetes: An Unresolved Issue.Nutrients. 2019 Mar 16;11(3):642. doi: 10.3390/nu11030642. Nutrients. 2019. PMID: 30884820 Free PMC article. Review.
References
-
- Nagpal S, Na S, Rathnachalam R (2005) Noncalcemic actions of vitamin D receptor ligands. Endocr Rev 26: 662–687. - PubMed
-
- Samuel S, Sitrin MD (2008) Vitamin D's role in cell proliferation and differentiation. Nutr Rev 66: S116–S124. - PubMed
-
- Blumberg JM, Tzameli I, Astapova I, Lam FS, Flier JS, et al. (2006) Complex role of the vitamin D receptor and its ligand in adipogenesis in 3T3-L1 cells. J Biol Chem 281: 11205–11213. - PubMed
-
- Kong J, Li YC (2006) Molecular mechanism of 1,25-dihydroxyvitamin D3 inhibition of adipogenesis in 3T3-L1 cells. Am J Physiol Endocrinol Metab 290: E916–E924. - PubMed
-
- Shi H, Norman AW, Okamura WH, Sen A, Zemel MB (2001) 1alpha,25-Dihydroxyvitamin D3 modulates human adipocyte metabolism via nongenomic action. FASEB J 15: 2751–2753. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

