CD-NP: a novel engineered dual guanylyl cyclase activator with anti-fibrotic actions in the heart
- PMID: 23272242
- PMCID: PMC3525541
- DOI: 10.1371/journal.pone.0052422
CD-NP: a novel engineered dual guanylyl cyclase activator with anti-fibrotic actions in the heart
Abstract
Natriuretic peptides (NPs) are cardioprotective through the activation of guanylyl cyclase (GC) receptors A and B. CD-NP, also known as cenderitide, is a novel engineered NP that was designed to uniquely serve as a first-in-class dual GC receptor agonist. Recognizing the aldosterone suppressing actions of GC-A activation and the potent inhibitory actions on collagen synthesis and fibroblast proliferation through GC-B activation, the current study was designed to establish the anti-fibrotic actions of CD-NP, administered subcutaneously, in an experimental rat model of early cardiac fibrosis induced by unilateral nephrectomy (UNX). Our results demonstrate that a two week subcutaneous infusion of CD-NP significantly suppresses left ventricular fibrosis and circulating aldosterone, while preserving both systolic and diastolic function, in UNX rats compared to vehicle treated UNX rats. Additionally we also confirmed, in vitro, that CD-NP significantly generates the second messenger, cGMP, through both the GC-A and GC-B receptors. Taken together, this novel dual GC receptor activator may represent an innovative anti-fibrotic therapeutic agent.
Conflict of interest statement
Figures

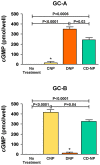
 P<0.05 vs UNX+Vehicle.
P<0.05 vs UNX+Vehicle.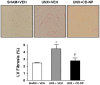
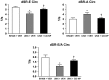
 P<0.05 vs UNX+Vehicle.
P<0.05 vs UNX+Vehicle.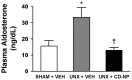
 P<0.05 vs UNX+Vehicle.
P<0.05 vs UNX+Vehicle.Similar articles
-
Cenderitide: structural requirements for the creation of a novel dual particulate guanylyl cyclase receptor agonist with renal-enhancing in vivo and ex vivo actions.Eur Heart J Cardiovasc Pharmacother. 2016 Apr;2(2):98-105. doi: 10.1093/ehjcvp/pvv040. Epub 2015 Dec 10. Eur Heart J Cardiovasc Pharmacother. 2016. PMID: 27340557 Free PMC article.
-
Cardiac fibrosis in end-stage human heart failure and the cardiac natriuretic peptide guanylyl cyclase system: regulation and therapeutic implications.J Mol Cell Cardiol. 2014 Oct;75:199-205. doi: 10.1016/j.yjmcc.2014.08.001. Epub 2014 Aug 9. J Mol Cell Cardiol. 2014. PMID: 25117468 Free PMC article.
-
CD-NP: an innovative designer natriuretic peptide activator of particulate guanylyl cyclase receptors for cardiorenal disease.Curr Heart Fail Rep. 2010 Sep;7(3):93-9. doi: 10.1007/s11897-010-0016-6. Curr Heart Fail Rep. 2010. PMID: 20582736 Review.
-
Design, synthesis, and actions of a novel chimeric natriuretic peptide: CD-NP.J Am Coll Cardiol. 2008 Jul 1;52(1):60-8. doi: 10.1016/j.jacc.2008.02.077. J Am Coll Cardiol. 2008. PMID: 18582636 Free PMC article.
-
Regulation and therapeutic targeting of peptide-activated receptor guanylyl cyclases.Pharmacol Ther. 2011 Apr;130(1):71-82. doi: 10.1016/j.pharmthera.2010.12.005. Epub 2010 Dec 24. Pharmacol Ther. 2011. PMID: 21185863 Free PMC article. Review.
Cited by
-
Natriuretic Peptide-Based Novel Therapeutics: Long Journeys of Drug Developments Optimized for Disease States.Biology (Basel). 2022 Jun 3;11(6):859. doi: 10.3390/biology11060859. Biology (Basel). 2022. PMID: 35741380 Free PMC article. Review.
-
Vasodilators in Acute Heart Failure: Review of the Latest Studies.Curr Emerg Hosp Med Rep. 2014 Jun;2(2):126-132. doi: 10.1007/s40138-014-0040-z. Curr Emerg Hosp Med Rep. 2014. PMID: 24855585 Free PMC article.
-
CRRL269: a novel designer and renal-enhancing pGC-A peptide activator.Am J Physiol Regul Integr Comp Physiol. 2018 Mar 1;314(3):R407-R414. doi: 10.1152/ajpregu.00286.2017. Epub 2017 Nov 29. Am J Physiol Regul Integr Comp Physiol. 2018. PMID: 29187381 Free PMC article.
-
C-type natriuretic peptide co-ordinates cardiac structure and function.Eur Heart J. 2020 Mar 1;41(9):1006-1020. doi: 10.1093/eurheartj/ehz093. Eur Heart J. 2020. PMID: 30903134 Free PMC article.
-
C-type Natriuretic Peptide: A Multifaceted Paracrine Regulator in the Heart and Vasculature.Int J Mol Sci. 2019 May 8;20(9):2281. doi: 10.3390/ijms20092281. Int J Mol Sci. 2019. PMID: 31072047 Free PMC article. Review.
References
-
- Potter LR, Abbey-Hosch S, Dickey DM (2006) Natriuretic peptides, their receptors, and cyclic guanosine monophosphate-dependent signaling functions. Endocr Rev 27: 47–72. - PubMed
-
- Koller KJ, Lowe DG, Bennett GL, Minamino N, Kangawa K, et al. (1991) Selective activation of the B natriuretic peptide receptor by C-type natriuretic peptide (CNP). Science 252: 120–123. - PubMed
-
- Suga S, Nakao K, Hosoda K, Mukoyama M, Ogawa Y, et al. (1992) Receptor selectivity of natriuretic peptide family, atrial natriuretic peptide, brain natriuretic peptide, and C-type natriuretic peptide. Endocrinology 130: 229–239. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

