Regulation of biotransformation systems and ABC transporters by benznidazole in HepG2 cells: involvement of pregnane X-receptor
- PMID: 23272261
- PMCID: PMC3521711
- DOI: 10.1371/journal.pntd.0001951
Regulation of biotransformation systems and ABC transporters by benznidazole in HepG2 cells: involvement of pregnane X-receptor
Abstract
Background: Benznidazole (BZL) is the only antichagasic drug available in most endemic countries. Its effect on the expression and activity of drug-metabolizing and transporter proteins has not been studied yet.
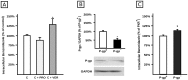
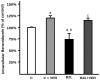
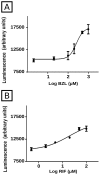
Methodology/principal findings: Expression and activity of P-glycoprotein (P-gp), Multidrug resistance-associated protein 2 (MRP2), Cytochrome P450 3A4 (CYP3A4), and Glutathione S-transferase (GST) were evaluated in HepG2 cells after treatment with BZL. Expression was estimated by immunoblotting and real time PCR. P-gp and MRP2 activities were estimated using model substrates rhodamine 123 and dinitrophenyl-S-glutathione (DNP-SG), respectively. CYP3A4 and GST activities were evaluated through their abilities to convert proluciferin into luciferin and 1-chloro-2,4-dinitrobenzene into DNP-SG, respectively. BZL (200 µM) increased the expression (protein and mRNA) of P-gp, MRP2, CYP3A4, and GSTπ class. A concomitant enhancement of activity was observed for all these proteins, except for CYP3A4, which exhibited a decreased activity. To elucidate if pregnane X receptor (PXR) mediates BZL response, its expression was knocked down with a specific siRNA. In this condition, the effect of BZL on P-gp, MRP2, CYP3A4, and GSTπ protein up-regulation was completely abolished. Consistent with this, BZL was able to activate PXR, as detected by reporter gene assay. Additional studies, using transporter inhibitors and P-gp-knock down cells, demonstrated that P-gp is involved in BZL extrusion. Pre-treatment of HepG2 cells with BZL increased its own efflux, as a consequence of P-gp up-regulation.
Conclusions/significance: Modifications in the activity of biotransformation and transport systems by BZL may alter the pharmacokinetics and efficiency of drugs that are substrates of these systems, including BZL itself.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures






Similar articles
-
Differential effects of the enantiomers of tamsulosin and tolterodine on P-glycoprotein and cytochrome P450 3A4.Naunyn Schmiedebergs Arch Pharmacol. 2017 Jan;390(1):49-59. doi: 10.1007/s00210-016-1304-9. Epub 2016 Sep 27. Naunyn Schmiedebergs Arch Pharmacol. 2017. PMID: 27678410
-
Modulation of biotransformation systems and ABC transporters by benznidazole in rats.Antimicrob Agents Chemother. 2013 Oct;57(10):4894-902. doi: 10.1128/AAC.02531-12. Epub 2013 Jul 22. Antimicrob Agents Chemother. 2013. PMID: 23877690 Free PMC article.
-
Transport of 5,5-diphenylbarbituric acid and its precursors and their effect on P-gp, MRP2 and CYP3A4 in Caco-2 and LS180 cells.Eur J Pharm Sci. 2011 Jan 18;42(1-2):19-29. doi: 10.1016/j.ejps.2010.10.001. Epub 2010 Oct 16. Eur J Pharm Sci. 2011. PMID: 20955791
-
Pregnane X receptor mediates the induction of P-glycoprotein by spironolactone in HepG2 cells.Toxicology. 2011 Jul 11;285(1-2):18-24. doi: 10.1016/j.tox.2011.03.015. Epub 2011 Apr 1. Toxicology. 2011. PMID: 21459122
-
Implication of metabolomics and transporter modulation based strategies to minimize multidrug resistance and enhance site-specific bioavailability: a needful consideration toward modern anticancer drug discovery.Drug Metab Rev. 2022 May;54(2):101-119. doi: 10.1080/03602532.2022.2048007. Epub 2022 Mar 7. Drug Metab Rev. 2022. PMID: 35254954 Review.
Cited by
-
Drug transporter and oxidative stress gene expression in human macrophages infected with benznidazole-sensitive and naturally benznidazole-resistant Trypanosoma cruzi parasites treated with benznidazole.Parasit Vectors. 2019 May 24;12(1):262. doi: 10.1186/s13071-019-3485-9. Parasit Vectors. 2019. PMID: 31126349 Free PMC article.
-
Pharmacokinetics of Benznidazole in Experimental Chronic Chagas Disease Using the Swiss Mouse-Berenice-78 Trypanosoma cruzi Strain Model.Antimicrob Agents Chemother. 2021 Jan 20;65(2):e01383-20. doi: 10.1128/AAC.01383-20. Print 2021 Jan 20. Antimicrob Agents Chemother. 2021. PMID: 33168611 Free PMC article.
-
Differential effects of the enantiomers of tamsulosin and tolterodine on P-glycoprotein and cytochrome P450 3A4.Naunyn Schmiedebergs Arch Pharmacol. 2017 Jan;390(1):49-59. doi: 10.1007/s00210-016-1304-9. Epub 2016 Sep 27. Naunyn Schmiedebergs Arch Pharmacol. 2017. PMID: 27678410
-
The phytoestrogens daidzein and equol inhibit the drug transporter BCRP/ABCG2 in breast cancer cells: potential chemosensitizing effect.Eur J Nutr. 2019 Feb;58(1):139-150. doi: 10.1007/s00394-017-1578-9. Epub 2017 Nov 3. Eur J Nutr. 2019. PMID: 29101532
-
Nanostructured lipid carriers containing benznidazole: physicochemical, biopharmaceutical and cellular in vitro studies.Beilstein J Nanotechnol. 2023 Jul 28;14:804-818. doi: 10.3762/bjnano.14.66. eCollection 2023. Beilstein J Nanotechnol. 2023. PMID: 37533841 Free PMC article.
References
-
- Rassi A Jr, Rassi A (2010) Chagas disease. Lancet 375: 1388–1402. - PubMed
-
- Gascón J, Albajar P, Cañas E, Flores M, Gómez i Prat J, et al. (2007) Diagnosis, management and treatment of chronic Chagas' heart disease in areas where Trypanosoma cruzi infection is not endemic. Rev Esp Cardiol 60: 285–293. - PubMed
-
- Rodriguez-Morales AJ, Benitez, Tellez I, Franco-Paredes C (2008) Chagas disease screening among Latin American immigrants in nonendemic settings. Travel Med Infect Dis 6: 162–163. - PubMed
-
- Aguilar EG, de Arranz CK, de Toranzo JA, Castro JA (1985) Benznidazole and nifurtimox nitroreductase activity in liver microsomes from male rats preinduced with phenobarbital or 3-methylcholanthrene. Res Commun Chem Pathol Pharmacol 50: 443–446. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

