Temporal changes in collagen cross-links in spontaneous articular cartilage repair
- PMID: 23272271
- PMCID: PMC3529722
- DOI: 10.1177/1947603512437736
Temporal changes in collagen cross-links in spontaneous articular cartilage repair
Abstract
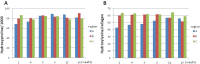
OBJECTIVE: Little is known about how the biochemical properties of collagen change during tissue regeneration following cartilage damage. In the current study, temporal changes in cartilage repair tissue biochemistry were assessed in a rabbit osteochondral defect. DESIGN: Bilateral full thickness 3mm osteochondral trochlear groove defects were created in 54 adult male skeletally mature New Zealand white rabbits and tissue repair monitored over 16 weeks. Collagen content, cross-links, lysyl hydroxylation, gene expression, histological grading, and FTIR analyses were performed at 2, 4, 6, 8, 12, and 16 weeks. RESULTS: Defect fill occurred at ~4 weeks post-injury, however, histological grading showed that the repair tissue never became normal, primarily due to the presence of fibrocartilage. Gene expression levels of Col1a1 and Col1a2 were higher in the defect compared to adjacent regions. Collagen content in the repair tissue reached the level of normal cartilage at 6 weeks, but it took 12 weeks for the extent of lysine hydroxylation to return to normal. Divalent immature cross-links markedly increased in the early stages of repair. Though the levels gradually diminished thereafter, they never returned to the normal levels. The mature cross-link, pyridinoline, gradually increased with time and nearly reached normal levels by week 16. Infrared imaging data of protein content paralleled the biochemical data. However, collagen maturity, a parameter previously shown to reflect collagen cross-link ratios in bone, did not correlate with the biochemical determination of cross-links in the repair tissue.. CONCLUSION: Collagen biochemical data could provide markers for clinical monitoring in a healing defect.
Conflict of interest statement
Figures




Similar articles
-
The maturity of tissue-engineered cartilage in vitro affects the repairability for osteochondral defect.Tissue Eng Part A. 2011 Dec;17(23-24):3057-65. doi: 10.1089/ten.TEA.2010.0605. Epub 2011 Oct 17. Tissue Eng Part A. 2011. PMID: 21736425 Free PMC article.
-
Repair of articular cartilage defects one year after treatment with recombinant human bone morphogenetic protein-2 (rhBMP-2).J Bone Joint Surg Am. 2000 Feb;82(2):151-60. doi: 10.2106/00004623-200002000-00001. J Bone Joint Surg Am. 2000. PMID: 10682724
-
Microfracture combined with osteochondral paste implantation was more effective than microfracture alone for full-thickness cartilage repair.Knee Surg Sports Traumatol Arthrosc. 2013 Aug;21(8):1770-6. doi: 10.1007/s00167-012-2031-5. Epub 2012 May 10. Knee Surg Sports Traumatol Arthrosc. 2013. PMID: 22572868
-
Evaluation of early osteochondral defect repair in a rabbit model utilizing fourier transform-infrared imaging spectroscopy, magnetic resonance imaging, and quantitative T2 mapping.Tissue Eng Part C Methods. 2010 Jun;16(3):355-64. doi: 10.1089/ten.TEC.2009.0020. Tissue Eng Part C Methods. 2010. PMID: 19586313 Free PMC article.
-
Collagen cross-links in mineralizing tissues: a review of their chemistry, function, and clinical relevance.Bone. 1998 Mar;22(3):181-7. doi: 10.1016/s8756-3282(97)00279-2. Bone. 1998. PMID: 9514209 Review.
Cited by
-
High reported rate of return to play following bone marrow stimulation for osteochondral lesions of the talus.Knee Surg Sports Traumatol Arthrosc. 2019 Sep;27(9):2721-2730. doi: 10.1007/s00167-018-4913-7. Epub 2018 Mar 26. Knee Surg Sports Traumatol Arthrosc. 2019. PMID: 29582098 Review.
-
Mesenchymal Stem/Progenitor Cells Derived from Articular Cartilage, Synovial Membrane and Synovial Fluid for Cartilage Regeneration: Current Status and Future Perspectives.Stem Cell Rev Rep. 2017 Oct;13(5):575-586. doi: 10.1007/s12015-017-9753-1. Stem Cell Rev Rep. 2017. PMID: 28721683 Review.
-
Clinical outcome of autologous chondrocyte implantation is correlated with infrared spectroscopic imaging-derived parameters.Osteoarthritis Cartilage. 2012 Sep;20(9):988-96. doi: 10.1016/j.joca.2012.05.007. Epub 2012 May 31. Osteoarthritis Cartilage. 2012. PMID: 22659601 Free PMC article.
-
Hyaluronan thiomer gel/matrix mediated healing of articular cartilage defects in New Zealand White rabbits-a pilot study.J Exp Orthop. 2017 Dec;4(1):14. doi: 10.1186/s40634-017-0089-1. Epub 2017 May 3. J Exp Orthop. 2017. PMID: 28470629 Free PMC article.
-
Monitoring the Progression of Spontaneous Articular Cartilage Healing with Infrared Spectroscopy.Cartilage. 2015 Jul;6(3):174-84. doi: 10.1177/1947603515572874. Cartilage. 2015. PMID: 26175863 Free PMC article.
References
-
- Alford JW, Cole BJ. Cartilage restoration, part 2: techniques, outcomes, and future directions. Am J Sports Med. 2005;33:443-60. - PubMed
-
- Alford JW, Cole BJ. Cartilage restoration, part 1. Am J Sports Med. 2005;33:295-306. - PubMed
-
- Anderson AF, Smith M. Progress in cartilage restoration. Am J Sports Med. 2009;37:7S-9S. - PubMed
-
- Moseley JB, O’Malley K, Petersen NJ, Menke TJ, Brody BA, Kuykendall DH, et al. A controlled trial of arthroscopic surgery for osteoarthritis of the knee. N Engl J Med. 2002;347:81-8. - PubMed
-
- Shiiba M, Arnaud SB, Tanzawa H, Kitamura E, Yamauchi M. Regional alterations of type I collagen in rat tibia induced by skeletal unloading. J Bone Miner Res. 2002;17:1639-45. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

