The Keap1-Nrf2 system in cancers: stress response and anabolic metabolism
- PMID: 23272301
- PMCID: PMC3530133
- DOI: 10.3389/fonc.2012.00200
The Keap1-Nrf2 system in cancers: stress response and anabolic metabolism
Abstract
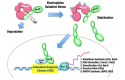
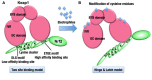
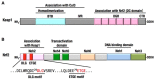
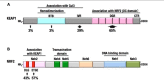
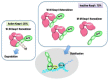
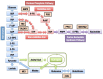
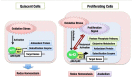
The Keap1-Nrf2 [Kelch-like ECH-associated protein 1-nuclear factor (erythroid-derived 2)-like 2] pathway plays a central role in the protection of cells against oxidative and xenobiotic stresses. Nrf2 is a potent transcription activator that recognizes a unique DNA sequence known as the antioxidant response element (ARE). Under normal conditions, Nrf2 binds to Keap1 in the cytoplasm, resulting in proteasomal degradation. Following exposure to electrophiles or reactive oxygen species, Nrf2 becomes stabilized, translocates into the nucleus, and activates the transcription of various cytoprotective genes. Increasing attention has been paid to the role of Nrf2 in cancer cells because the constitutive stabilization of Nrf2 has been observed in many human cancers with poor prognosis. Recent studies have shown that the antioxidant and detoxification activities of Nrf2 confer chemo- and radio-resistance to cancer cells. In this review, we provide an overview of the Keap1-Nrf2 system and discuss its role under physiological and pathological conditions, including cancers. We also introduce the results of our recent study describing Nrf2 function in the metabolism of cancer cells. Nrf2 likely confers a growth advantage to cancer cells through enhancing cytoprotection and anabolism. Finally, we discuss the possible impact of Nrf2 inhibitors on cancer therapy.
Keywords: glutathione; purine nucleotide; redox homeostasis; stress response; transcription.
Figures

References
-
- Aoki Y., Sato H., Nishimura N., Takahashi S., Itoh K., Yamamoto M. (2001). Accelerated DNA adduct formation in the lung of the Nrf2 knockout mouse exposed to diesel exhaust. Toxicol. Appl. Pharmacol. 173 154–160 - PubMed
-
- Bea F., Hudson F. N., Chait A., Kavanagh T. J., Rosenfeld M. E. (2003). Induction of glutathione synthesis in macrophages by oxidized low-density lipoproteins is mediated by consensus antioxidant response elements. Circ. Res. 92 386–393 - PubMed
-
- Boros L. G., Puigjaner J., Cascante M., Lee W. N., Brandes J. L., Bassilian S., et al. (1997). Oxythiamine and dehydroepiandrosterone inhibit the nonoxidative synthesis of ribose and tumor cell proliferation. Cancer Res. 57 4242–4248 - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

