Amygdala activity contributes to the dissociative effect of cannabis on pain perception
- PMID: 23273106
- PMCID: PMC3549497
- DOI: 10.1016/j.pain.2012.09.017
Amygdala activity contributes to the dissociative effect of cannabis on pain perception
Abstract
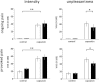
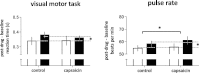
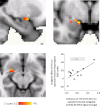
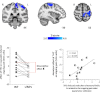
Cannabis is reported to be remarkably effective for the relief of otherwise intractable pain. However, the bases for pain relief afforded by this psychotropic agent are debatable. Nonetheless, the frontal-limbic distribution of cannabinoid receptors in the brain suggests that cannabis may target preferentially the affective qualities of pain. This central mechanism of action may be relevant to cannabinoid analgesia in humans, but has yet to be demonstrated. Here, we employed functional magnetic resonance imaging to investigate the effects of delta-9-tetrahydrocannabinol (THC), a naturally occurring cannabinoid, on brain activity related to cutaneous ongoing pain and hyperalgesia that were temporarily induced by capsaicin in healthy volunteers. On average, THC reduced the reported unpleasantness, but not the intensity of ongoing pain and hyperalgesia: the specific analgesic effect on hyperalgesia was substantiated by diminished activity in the anterior mid cingulate cortex. In individuals, the drug-induced reduction in the unpleasantness of hyperalgesia was positively correlated with right amygdala activity. THC also reduced functional connectivity between the amygdala and primary sensorimotor areas during the ongoing-pain state. Critically, the reduction in sensory-limbic functional connectivity was positively correlated with the difference in drug effects on the unpleasantness and the intensity of ongoing pain. Peripheral mechanisms alone cannot account for the dissociative effects of THC on the pain that was observed. Instead, the data reveal that amygdala activity contributes to interindividual response to cannabinoid analgesia, and suggest that dissociative effects of THC in the brain are relevant to pain relief in humans.
Copyright © 2012 International Association for the Study of Pain. Published by Elsevier B.V. All rights reserved.
Figures


References
-
- Abrams D.I., Jay C.A., Shade S.B., Vizoso H., Reda H., Press S., Kelly M.E., Rowbotham M.C., Petersen K.L. Cannabis in painful HIV-associated sensory neuropathy: a randomized placebo-controlled trial. Neurology. 2007;68:515–521. - PubMed
-
- Albe-Fessard D., Berkley K.J., Kruger L., Ralston H.J., III, Willis W.D., Jr. Diencephalic mechanisms of pain sensation. Brain Res. 1985;356:217–296. - PubMed
-
- Amaral D.G., Price J.L. Amygdalo-cortical projections in the monkey (Macaca fascicularis) J Comp Neurol. 1984;230:465–496. - PubMed
-
- Beaulieu P., Ware M. Reassessment of the role of cannabinoids in the management of pain. Curr Opin Anaesthesiol. 2007;20:473–477. - PubMed
-
- Beckmann C.F., Jenkinson M., Smith S.M. General multilevel linear modeling for group analysis in FMRI. Neuroimage. 2003;20:1052–1063. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

