Potential impact of a nine-valent vaccine in human papillomavirus related cervical disease
- PMID: 23273245
- PMCID: PMC3554470
- DOI: 10.1186/1750-9378-7-38
Potential impact of a nine-valent vaccine in human papillomavirus related cervical disease
Abstract
Background: Information on human papillomavirus (HPV) type distribution is necessary to evaluate the potential impact of current and future HPV vaccines. We estimated the relative contribution (RC) to invasive cervical cancer (ICC) and precancerous cervical lesions of the nine HPV types (HPV 6/11/16/18/31/33/45/52/58) included in an HPV vaccine currently under development.
Methods: Estimations on ICC were based on an international study of 8,977 HPV positive cases and estimations on precancerous cervical lesions were extracted from a published meta-analysis including 115,789 HPV positive women. Globocan 2008 and 2010 World Population Prospects were used to estimate current and future projections of new ICC cases.
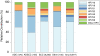
Results: RC of the 9 HPV types in ICC was 89.4%, with 18.5% of cases positive for HPV 31/33/45/52/58. Regional variations were observed. RCs varied by histology, ranging between 89.1% in squamous cell carcinomas (SCC) and 95.5% in adenocarcinomas (ADC). HPV 16/18/45 were detected in 94.2% of ADC. RC of the 9 types altogether decreased with age (trend test p < 0.0001), driven by the decrease in older ages of HPV 16/18/45. In contrast, the RC of HPV 31/33/52/58 increased with age. Due to population growth alone, projected estimates of ICC cases attributable to the 9 types are expected to rise from 493,770 new cases in 2012 to 560,887 new cases in 2025.The RCs of individual high risk HPV types varied by cytological and histological grades of HPV-positive precancerous cervical lesions, and there was an under representation of HPV 18 and 45 compared to ICC.
Conclusions: The addition of HPV 31/33/45/52/58 to HPV types included in current vaccines could prevent almost 90% of ICC cases worldwide. If the nine-valent vaccine achieves the same degree of efficacy than previous vaccines, world incidence rates could be substantially reduced.
Figures


References
-
- Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. GLOBOCAN 2008 v1.2, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 10 [Internet] International Agency for Research on Cancer, Lyon, France; 2010. Available at: http://globocan.iarc.fr [Accessed on April 2012]
-
- Walboomers JM, Jacobs MV, Manos MM, Bosch FX, Kummer JA, Shah KV, Snijders PJ, Peto J, Meijer CJ, Muñoz N. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999;189(1):12–19. doi: 10.1002/(SICI)1096-9896(199909)189:1<12::AID-PATH431>3.0.CO;2-F. - DOI - PubMed
-
- IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Vol 100, A Review of Human Carcinogens. Part B: Biological Agents. Lyon, France: International Agency for Research on Cancer; 2011.
-
- World Health Organization (WHO) Human papillomavirus vaccines WHO position paper. Geneva: World Health Organization; 2009. Available at: http://www.who.int/wer/2009/wer8415.pdf [Accessed on April 2012]
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

