Traumatic brain injury in aged animals increases lesion size and chronically alters microglial/macrophage classical and alternative activation states
- PMID: 23273602
- PMCID: PMC3572914
- DOI: 10.1016/j.neurobiolaging.2012.11.013
Traumatic brain injury in aged animals increases lesion size and chronically alters microglial/macrophage classical and alternative activation states
Abstract
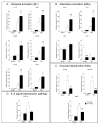
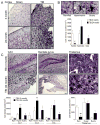
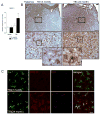
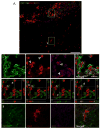
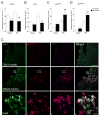
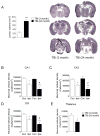
Traumatic brain injury (TBI) causes chronic microglial activation that contributes to subsequent neurodegeneration, with clinical outcomes declining as a function of aging. Microglia/macrophages (MG/Mɸ) have multiple phenotypes, including a classically activated, proinflammatory (M1) state that might contribute to neurotoxicity, and an alternatively activated (M2) state that might promote repair. In this study we used gene expression, immunohistochemical, and stereological analyses to show that TBI in aged versus young mice caused larger lesions associated with an M1/M2 balance switch and increased numbers of reactive (bushy and hypertrophic) MG/Mɸ in the cortex, hippocampus, and thalamus. Chitinase3-like 3 (Ym1), an M2 phenotype marker, displayed heterogeneous expression after TBI with amoeboid-like Ym1-positive MG/Mɸ at the contusion site and ramified Ym1-positive MG/Mɸ at distant sites; this distribution was age-related. Aged-injured mice also showed increased MG/Mɸ expression of major histocompatibility complex II and NADPH oxidase, and reduced antioxidant enzyme expression which was associated with lesion size and neurodegeneration. Thus, altered relative M1/M2 activation and an nicotinamide adenine dinucleotide phosphate oxidase (NADPH oxidase)-mediated shift in redox state might contribute to worse outcomes observed in older TBI animals by creating a more proinflammatory M1 MG/Mɸ activation state.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures


References
-
- Ansari MA, Roberts KN, Scheff SW. A time course of contusion-induced oxidative stress and synaptic proteins in cortex in a rat model of TBI. Journal of neurotrauma. 2008b;25:513–26. - PubMed
-
- Badan I, Buchhold B, Hamm A, Gratz M, Walker LC, Platt D, Kessler C, Popa-Wagner A. Accelerated glial reactivity to stroke in aged rats correlates with reduced functional recovery. J Cereb Blood Flow Metab. 2003;23:845–54. - PubMed
-
- Block ML, Hong JS. Microglia and inflammation-mediated neurodegeneration: multiple triggers with a common mechanism. Prog Neurobiol. 2005;76:77–98. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

