New insights into mechanisms of stem cell daughter fate determination in regenerative tissues
- PMID: 23273858
- PMCID: PMC5788169
- DOI: 10.1016/B978-0-12-405210-9.00001-1
New insights into mechanisms of stem cell daughter fate determination in regenerative tissues
Abstract
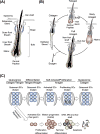
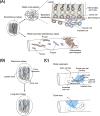
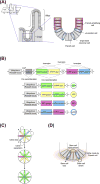
Stem cells can self-renew and differentiate over extended periods of time. Understanding how stem cells acquire their fates is a central question in stem cell biology. Early work in Drosophila germ line and neuroblast showed that fate choice is achieved by strict asymmetric divisions that can generate each time one stem and one differentiated cell. More recent work suggests that during homeostasis, some stem cells can divide symmetrically to generate two differentiated cells or two identical stem cells to compensate for stem cell loss that occurred by direct differentiation or apoptosis. The interplay of all these factors ensures constant tissue regeneration and the maintenance of stem cell pool size. This interplay can be modeled as a population-deterministic dynamics that, at least in some systems, may be described as stochastic behavior. Here, we overview recent progress made on the characterization of stem cell dynamics in regenerative tissues.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures
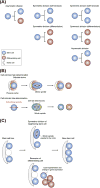
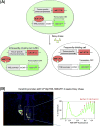
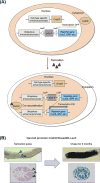
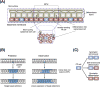
References
-
- Ahmed S. The culture of neural stem cells. J. Cell. Biochem. 2009;106:1–6. - PubMed
-
- Altman J, Das GD. Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J. Comp. Neurol. 1965;124:319–335. - PubMed
-
- Ambler CA, Maatta A. Epidermal stem cells: location, potential and contribution to cancer. J. Pathol. 2009;217:206–216. - PubMed
-
- Bardin AJ, et al. Asymmetric localization and function of cell-fate determinants: a fly’s view. Curr. Opin. Neurobiol. 2004;14:6–14. - PubMed
-
- Barker N, et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003–1007. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

