Sphingosine-1-phosphate links persistent STAT3 activation, chronic intestinal inflammation, and development of colitis-associated cancer
- PMID: 23273921
- PMCID: PMC3578577
- DOI: 10.1016/j.ccr.2012.11.013
Sphingosine-1-phosphate links persistent STAT3 activation, chronic intestinal inflammation, and development of colitis-associated cancer
Abstract
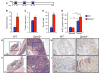
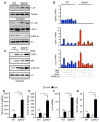
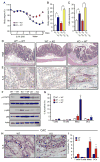
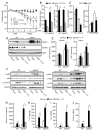
Inflammatory bowel disease is an important risk factor for colorectal cancer. We show that sphingosine-1-phosphate (S1P) produced by upregulation of sphingosine kinase 1 (SphK1) links chronic intestinal inflammation to colitis-associated cancer (CAC) and both are exacerbated by deletion of Sphk2. S1P is essential for production of the multifunctional NF-κB-regulated cytokine IL-6, persistent activation of the transcription factor STAT3, and consequent upregulation of the S1P receptor, S1PR1. The prodrug FTY720 decreased SphK1 and S1PR1 expression and eliminated the NF-κB/IL-6/STAT3 amplification cascade and development of CAC, even in Sphk2(-/-) mice, and may be useful in treating colon cancer in individuals with ulcerative colitis. Thus, the SphK1/S1P/S1PR1 axis is at the nexus between NF-κB and STAT3 and connects chronic inflammation and CAC.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures



Comment in
-
Sphingosine 1-phosphate is a missing link between chronic inflammation and colon cancer.Cancer Cell. 2013 Jan 14;23(1):5-7. doi: 10.1016/j.ccr.2012.12.005. Cancer Cell. 2013. PMID: 23328479
-
Tumorigenesis: All together now.Nat Rev Cancer. 2013 Mar;13(3):148. doi: 10.1038/nrc3469. Epub 2013 Jan 24. Nat Rev Cancer. 2013. PMID: 23344496 No abstract available.
References
-
- Becker C, Fantini MC, Schramm C, Lehr HA, Wirtz S, Nikolaev A, Burg J, Strand S, Kiesslich R, Huber S, et al. TGF-beta suppresses tumor progression in colon cancer by inhibition of IL-6 trans-signaling. Immunity. 2004;21:491–501. - PubMed
-
- Ben-Neriah Y, Karin M. Inflammation meets cancer, with NF-kappaB as the matchmaker. Nat Immunol. 2011;12:715–723. - PubMed
-
- Billich A, Bornancin F, Devay P, Mechtcheriakova D, Urtz N, Baumruker T. Phosphorylation of the imunomodulatory drug FTY720 by sphingosine kinases. J Biol Chem. 2003;278:47408–47415. - PubMed
-
- Bollrath J, Phesse TJ, von Burstin VA, Putoczki T, Bennecke M, Bateman T, Nebelsiek T, Lundgren-May T, Canli O, Schwitalla S, et al. gp130-mediated STAT3 activation in enterocytes regulates cell survival and cell-cycle progression during colitis-associated tumorigenesis. Cancer Cell. 2009;15:91–102. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- K12HD055881/HD/NICHD NIH HHS/United States
- R01CA61774/CA/NCI NIH HHS/United States
- P30 NS047463/NS/NINDS NIH HHS/United States
- K12 HD055881/HD/NICHD NIH HHS/United States
- R01 CA160688/CA/NCI NIH HHS/United States
- U19AI077435/AI/NIAID NIH HHS/United States
- P30 CA016059/CA/NCI NIH HHS/United States
- 5P30NS047463/NS/NINDS NIH HHS/United States
- T32 HL094290/HL/NHLBI NIH HHS/United States
- R37GM043880/GM/NIGMS NIH HHS/United States
- T32HL094290/HL/NHLBI NIH HHS/United States
- U19 AI077435/AI/NIAID NIH HHS/United States
- P30CA16059/CA/NCI NIH HHS/United States
- R01 CA061774/CA/NCI NIH HHS/United States
- R37 GM043880/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

