Comparison of auditory-vocal interactions across multiple types of vocalizations in marmoset auditory cortex
- PMID: 23274315
- PMCID: PMC3602939
- DOI: 10.1152/jn.00698.2012
Comparison of auditory-vocal interactions across multiple types of vocalizations in marmoset auditory cortex
Abstract
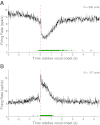
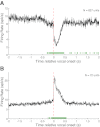
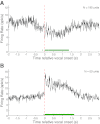
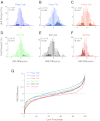
Auditory-vocal interaction, the modulation of auditory sensory responses during vocal production, is an important but poorly understood neurophysiological phenomenon in nonhuman primates. This sensory-motor processing has important behavioral implications for self-monitoring during vocal production as well as feedback-mediated vocal control for both animals and humans. Previous studies in marmosets have shown that a large portion of neurons in the auditory cortex are suppressed during self-produced vocalization but have primarily focused on a single type of isolation vocalization. The present study expands previous analyses to compare auditory-vocal interaction of cortical responses between different types of vocalizations. We recorded neurons from the auditory cortex of unrestrained marmoset monkeys with implanted electrode arrays and showed that auditory-vocal interactions generalize across vocalization types. We found the following: 1) Vocal suppression and excitation are a general phenomenon, occurring for all four major vocalization types. 2) Within individual neurons, suppression was the more general response, occurring for multiple vocalization types, while excitation tended to be more specific to a single vocalization type. 3) A subset of neurons changed their responses between different types of vocalization, most often from strong suppression or excitation for one vocalization to unresponsive for another, and only rarely from suppression to excitation. 4) Differences in neural responses between vocalization types were weakly correlated with passive response properties, measured by playbacks of acoustic stimuli including recorded vocalizations. These results indicate that vocalization-induced modulation of the auditory cortex is a general phenomenon applicable to all vocalization types, but variations within individual neurons suggest possible vocalization-specific coding.
Figures












Similar articles
-
Contributions of sensory tuning to auditory-vocal interactions in marmoset auditory cortex.Hear Res. 2017 May;348:98-111. doi: 10.1016/j.heares.2017.03.001. Epub 2017 Mar 9. Hear Res. 2017. PMID: 28284736 Free PMC article.
-
Sensory-motor interaction in the primate auditory cortex during self-initiated vocalizations.J Neurophysiol. 2003 Apr;89(4):2194-207. doi: 10.1152/jn.00627.2002. Epub 2002 Dec 11. J Neurophysiol. 2003. PMID: 12612021
-
Dynamics of auditory-vocal interaction in monkey auditory cortex.Cereb Cortex. 2005 Oct;15(10):1510-23. doi: 10.1093/cercor/bhi030. Epub 2005 Feb 2. Cereb Cortex. 2005. PMID: 15689521
-
Corollary Discharge Mechanisms During Vocal Production in Marmoset Monkeys.Biol Psychiatry Cogn Neurosci Neuroimaging. 2019 Sep;4(9):805-812. doi: 10.1016/j.bpsc.2019.06.008. Epub 2019 Jun 29. Biol Psychiatry Cogn Neurosci Neuroimaging. 2019. PMID: 31420219 Free PMC article. Review.
-
Auditory plasticity: vocal output shapes auditory cortex.Curr Biol. 2005 Jul 12;15(13):R503-5. doi: 10.1016/j.cub.2005.06.027. Curr Biol. 2005. PMID: 16005282 Review.
Cited by
-
Functional magnetic resonance imaging of auditory cortical fields in awake marmosets.Neuroimage. 2017 Nov 15;162:86-92. doi: 10.1016/j.neuroimage.2017.08.052. Epub 2017 Aug 19. Neuroimage. 2017. PMID: 28830766 Free PMC article.
-
Echolocation-related reversal of information flow in a cortical vocalization network.Nat Commun. 2022 Jun 25;13(1):3642. doi: 10.1038/s41467-022-31230-6. Nat Commun. 2022. PMID: 35752629 Free PMC article.
-
Orbitofrontal Cortex Neurons Respond to Sound and Activate Primary Auditory Cortex Neurons.Cereb Cortex. 2018 Mar 1;28(3):868-879. doi: 10.1093/cercor/bhw409. Cereb Cortex. 2018. PMID: 28069762 Free PMC article.
-
Responses of neurons in the marmoset primary auditory cortex to interaural level differences: comparison of pure tones and vocalizations.Front Neurosci. 2015 Apr 20;9:132. doi: 10.3389/fnins.2015.00132. eCollection 2015. Front Neurosci. 2015. PMID: 25941469 Free PMC article.
-
Multiple processes of vocal sensory-motor interaction in primate auditory cortex.Nat Commun. 2024 Apr 10;15(1):3093. doi: 10.1038/s41467-024-47510-2. Nat Commun. 2024. PMID: 38600118 Free PMC article.
References
-
- Békèsy G. The structure of the middle ear and the hearing of one's own voice by bone conduction. J Acoust Soc Am 21: 217–232, 1949
-
- Brumm H, Voss K, Kollmer I, Todt D. Acoustic communication in noise: regulation of call characteristics in a New World monkey. J Exp Biol 207: 443–448, 2004 - PubMed
-
- Burnett TA, Freedland MB, Larson CR, Hain TC. Voice F0 responses to manipulations in pitch feedback. J Acoust Soc Am 103: 3153–3161, 1998 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

