Risk of presentation to hospital with epileptic seizures after vaccination with monovalent AS03 adjuvanted pandemic A/H1N1 2009 influenza vaccine (Pandemrix): self controlled case series study
- PMID: 23274350
- PMCID: PMC3532724
- DOI: 10.1136/bmj.e7594
Risk of presentation to hospital with epileptic seizures after vaccination with monovalent AS03 adjuvanted pandemic A/H1N1 2009 influenza vaccine (Pandemrix): self controlled case series study
Abstract
Objective: To assess the risk of epileptic seizures in people with and without epilepsy after vaccination with a monovalent AS03 adjuvanted pandemic A/H1N1 influenza vaccine (Pandemrix; Glaxo SmithKline, Sweden).
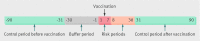
Design: Register based self controlled case series.
Setting: Three Swedish counties (source population 750,000).
Participants: 373,398 people (age 0-106, median 41.2) who were vaccinated. Vaccinated people with epileptic seizures, diagnosed as inpatients or outpatients, at any time from 90 days before until 90 days after any dose of vaccine.
Main outcome measures: Endpoints were admission to hospital or outpatient hospital care with epileptic seizures as the main diagnosis. The effect estimate of relative incidence was calculated as the incidence of epileptic seizures in period after exposure relative to the incidence of epileptic seizures in two control periods, one before and one after vaccination.
Results: 859 people experienced epileptic seizures during the study period. There was no increased risk of seizures in people with previously diagnosed epilepsy (relative incidence 1.01, 95% confidence interval 0.74 to 1.39) and a non-significant decrease in risk for people without epilepsy (0.67, 0.27 to 1.65) during the day 1-7 risk period (where day 1 is the day of vaccination). In a second risk period (day 8-30), there was a non-significant increased risk of seizures in people without epilepsy (1.11, 0.73 to 1.70) but no increase in risk for those with epilepsy (1.00, 0.83 to 1.21).
Conclusions: This study found no evidence of an increase in risk of presentation to hospital with epileptic seizures after vaccination with a monovalent AS03 adjuvanted pandemic H1N1 influenza vaccine.
Conflict of interest statement
Competing interests: All authors have completed the ICMJE uniform disclosure form at
Figures
Similar articles
-
Long term effectiveness of adjuvanted influenza A(H1N1)pdm09 vaccine in children.Vaccine. 2015 May 21;33(22):2558-61. doi: 10.1016/j.vaccine.2015.04.011. Epub 2015 Apr 11. Vaccine. 2015. PMID: 25869891
-
Change in risk for narcolepsy over time and impact of definition of onset date following vaccination with AS03 adjuvanted pandemic A/H1N1 influenza vaccine (Pandemrix) during the 2009 H1N1 influenza pandemic.Pharmacoepidemiol Drug Saf. 2019 Aug;28(8):1045-1053. doi: 10.1002/pds.4788. Epub 2019 May 6. Pharmacoepidemiol Drug Saf. 2019. PMID: 31062443
-
Febrile seizures after 2009 influenza A (H1N1) vaccination and infection: a nationwide registry-based study.BMC Infect Dis. 2015 Nov 9;15:506. doi: 10.1186/s12879-015-1263-7. BMC Infect Dis. 2015. PMID: 26553258 Free PMC article.
-
Narcolepsy, 2009 A(H1N1) pandemic influenza, and pandemic influenza vaccinations: what is known and unknown about the neurological disorder, the role for autoimmunity, and vaccine adjuvants.J Autoimmun. 2014 May;50:1-11. doi: 10.1016/j.jaut.2014.01.033. Epub 2014 Feb 19. J Autoimmun. 2014. PMID: 24559657 Review.
-
Narcolepsy and Pandemic Influenza Vaccination: What We Need to Know to be Ready for the Next Pandemic.Pediatr Infect Dis J. 2019 Aug;38(8):873-876. doi: 10.1097/INF.0000000000002398. Pediatr Infect Dis J. 2019. PMID: 31306400 Review.
Cited by
-
The Influence of Vaccine on Febrile Seizure.Curr Neuropharmacol. 2018;16(1):59-65. doi: 10.2174/1570159X15666170726115639. Curr Neuropharmacol. 2018. PMID: 28745219 Free PMC article. Review.
-
Effect of the adjuvanted (AS03) A/H1N1 2009 pandemic influenza vaccine on the risk of rejection in solid organ transplant recipients in England: a self-controlled case series.BMJ Open. 2016 Jan 28;6(1):e009264. doi: 10.1136/bmjopen-2015-009264. BMJ Open. 2016. PMID: 26823177 Free PMC article.
-
Impact of COVID-19 vaccine on epilepsy in adult subjects: an Italian multicentric experience.Neurol Sci. 2022 Aug;43(8):4627-4634. doi: 10.1007/s10072-022-06100-0. Epub 2022 May 2. Neurol Sci. 2022. PMID: 35501537 Free PMC article.
-
Safety Assessment of Influenza Vaccination for Neurological Outcomes Among Older Adults in Japan: A Self-Controlled Case Series Study.Pharmacoepidemiol Drug Saf. 2025 Jan;34(1):e70082. doi: 10.1002/pds.70082. Pharmacoepidemiol Drug Saf. 2025. PMID: 39777941 Free PMC article.
-
Adjuvanted (AS03) A/H1N1 2009 Pandemic Influenza Vaccines and Solid Organ Transplant Rejection: Systematic Signal Evaluation and Lessons Learnt.Drug Saf. 2017 Aug;40(8):693-702. doi: 10.1007/s40264-017-0532-3. Drug Saf. 2017. PMID: 28417321 Free PMC article.
References
-
- Madsen KM, Hviid A, Vestergaard M, Schendel D, Wohlfahrt J, Thorsen P, et al. A population-based study of measles, mumps, and rubella vaccination and autism. N Engl J Med 2002;347:1477-82. - PubMed
-
- Brown NJ, Berkovic SF, Scheffer IE. Vaccination, seizures and ‘vaccine damage’. Curr Opin Neurol 2007;20:181-7. - PubMed
-
- European Medicines Agency. Thirteenth pandemic pharmacovigilance update. www.ema.europa.eu/docs/en_GB/document_library/Report/2010/03/WC500075456....
-
- Schonberger LB, Bregman DJ, Sullivan-Bolyai JZ, Keenlyside RA, Ziegler DW, Retailliau HF, et al. Guillain-Barre syndrome following vaccination in the National Influenza Immunization Program, United States, 1976-1977. Am J Epidemiol 1979;110:105-23. - PubMed