DGKE variants cause a glomerular microangiopathy that mimics membranoproliferative GN
- PMID: 23274426
- PMCID: PMC3582208
- DOI: 10.1681/ASN.2012090903
DGKE variants cause a glomerular microangiopathy that mimics membranoproliferative GN
Abstract
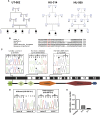
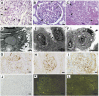
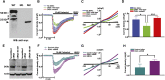
Renal microangiopathies and membranoproliferative GN (MPGN) can manifest similar clinical presentations and histology, suggesting the possibility of a common underlying mechanism in some cases. Here, we performed homozygosity mapping and whole exome sequencing in a Turkish consanguineous family and identified DGKE gene variants as the cause of a membranoproliferative-like glomerular microangiopathy. Furthermore, we identified two additional DGKE variants in a cohort of 142 unrelated patients diagnosed with membranoproliferative GN. This gene encodes the diacylglycerol kinase DGKε, which is an intracellular lipid kinase that phosphorylates diacylglycerol to phosphatidic acid. Immunofluorescence confocal microscopy demonstrated that mouse and rat Dgkε colocalizes with the podocyte marker WT1 but not with the endothelial marker CD31. Patch-clamp experiments in human embryonic kidney (HEK293) cells showed that DGKε variants affect the intracellular concentration of diacylglycerol. Taken together, these results not only identify a genetic cause of a glomerular microangiopathy but also suggest that the phosphatidylinositol cycle, which requires DGKE, is critical to the normal function of podocytes.
Figures
Comment in
-
Lipid kinase mutations in heritable glomerular microangiopathy.J Am Soc Nephrol. 2013 Feb;24(3):329-30. doi: 10.1681/ASN.2013010078. Epub 2013 Feb 14. J Am Soc Nephrol. 2013. PMID: 23411781 No abstract available.
References
-
- Braun MC, Licht C, Strife CF: Membranoproliferative glomerulonephritis. In: Pediatric Nephrology, edited by Avner ED, Harmon WH, Niaudet P, Yoshikawa N, Berlin, Springer, 2009, pp 783–797
-
- Sethi S, Fervenza FC: Membranoproliferative glomerulonephritis: Pathogenetic heterogeneity and proposal for a new classification. Semin Nephrol 31: 341–348, 2011 - PubMed
-
- Bakkaloglu A, Söylemezoglu O, Tinaztepe K, Saatçi U, Söylemezoglu F: Familial membranoproliferative glomerulonephritis. Nephrol Dial Transplant 10: 21–24, 1995 - PubMed
-
- Berry PL, McEnery PT, McAdams AJ, West CD: Membranoproliferative glomerulonephritis in two sibships. Clin Nephrol 16: 101–106, 1981 - PubMed
-
- Bogdanović RM, Dimitrijević JZ, Nikolić VN, Ognjanović MV, Rodić BD, Slavković BV: Membranoproliferative glomerulonephritis in two siblings: Report and literature review. Pediatr Nephrol 14: 400–405, 2000 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- RC4-DK090947/DK/NIDDK NIH HHS/United States
- DK1068306/DK/NIDDK NIH HHS/United States
- P30DK79328/DK/NIDDK NIH HHS/United States
- HHMI/Howard Hughes Medical Institute/United States
- 1R01DK090326-01A1/DK/NIDDK NIH HHS/United States
- P30 DK079328/DK/NIDDK NIH HHS/United States
- R01 DK090326/DK/NIDDK NIH HHS/United States
- DK85726/DK/NIDDK NIH HHS/United States
- CA095463/CA/NCI NIH HHS/United States
- DK1069274/DK/NIDDK NIH HHS/United States
- R01 CA095463/CA/NCI NIH HHS/United States
- R01 DK085726/DK/NIDDK NIH HHS/United States
- P30DK079328-04/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

