Azole affinity of sterol 14α-demethylase (CYP51) enzymes from Candida albicans and Homo sapiens
- PMID: 23274672
- PMCID: PMC3591892
- DOI: 10.1128/AAC.02067-12
Azole affinity of sterol 14α-demethylase (CYP51) enzymes from Candida albicans and Homo sapiens
Abstract
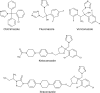
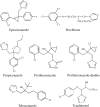
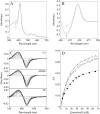
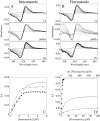
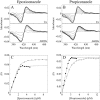
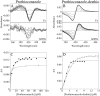
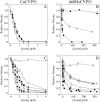
Candida albicans CYP51 (CaCYP51) (Erg11), full-length Homo sapiens CYP51 (HsCYP51), and truncated Δ60HsCYP51 were expressed in Escherichia coli and purified to homogeneity. CaCYP51 and both HsCYP51 enzymes bound lanosterol (K(s), 14 to 18 μM) and catalyzed the 14α-demethylation of lanosterol using Homo sapiens cytochrome P450 reductase and NADPH as redox partners. Both HsCYP51 enzymes bound clotrimazole, itraconazole, and ketoconazole tightly (dissociation constants [K(d)s], 42 to 131 nM) but bound fluconazole (K(d), ~30,500 nM) and voriconazole (K(d), ~2,300 nM) weakly, whereas CaCYP51 bound all five medical azole drugs tightly (K(d)s, 10 to 56 nM). Selectivity for CaCYP51 over HsCYP51 ranged from 2-fold (clotrimazole) to 540-fold (fluconazole) among the medical azoles. In contrast, selectivity for CaCYP51 over Δ60HsCYP51 with agricultural azoles ranged from 3-fold (tebuconazole) to 9-fold (propiconazole). Prothioconazole bound extremely weakly to CaCYP51 and Δ60HsCYP51, producing atypical type I UV-visible difference spectra (K(d)s, 6,100 and 910 nM, respectively), indicating that binding was not accomplished through direct coordination with the heme ferric ion. Prothioconazole-desthio (the intracellular derivative of prothioconazole) bound tightly to both CaCYP51 and Δ60HsCYP51 (K(d), ~40 nM). These differences in binding affinities were reflected in the observed 50% inhibitory concentration (IC(50)) values, which were 9- to 2,000-fold higher for Δ60HsCYP51 than for CaCYP51, with the exception of tebuconazole, which strongly inhibited both CYP51 enzymes. In contrast, prothioconazole weakly inhibited CaCYP51 (IC(50), ~150 μM) and did not significantly inhibit Δ60HsCYP51.
Figures
References
-
- Kelly SL, Lamb DC, Jackson CJ, Warrilow AGS, Kelly DE. 2003. The biodiversity of microbial cytochromes P450. Adv. Microb. Physiol. 47:131–186 - PubMed
-
- Lamb DC, Kelly DE, Waterman MR, Stromstedt M, Rozman D, Kelly SL. 1999. Characteristics of the heterologously expressed human lanosterol 14α-demethylase (other names: P45014DM, CYP51, P45051) and inhibition of the purified human and Candida albicans CYP51 with azole antifungal agents. Yeast 15:755–763 - PubMed
-
- Jefcoate CR, Gaylor JL, Calabrese RL. 1969. Ligand interactions with cytochrome P450. I. Binding of primary amines. Biochemistry 8:3455–3463 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

