Leptin regulates amyloid β production via the γ-secretase complex
- PMID: 23274884
- PMCID: PMC3557588
- DOI: 10.1016/j.bbadis.2012.12.009
Leptin regulates amyloid β production via the γ-secretase complex
Abstract
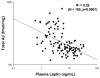
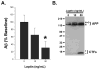
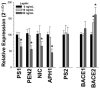
Alzheimer's disease (AD) is the most common age-related neurodegenerative disease, affecting an estimated 5.3million people in the United States. While many factors likely contribute to AD progression, it is widely accepted that AD is driven by the accumulation of β-amyloid (Aβ), a small, fibrillogenic peptide generated by the sequential proteolysis of the amyloid precursor protein by the β- and γ-secretases. Though the underlying causes of Aβ accumulation in sporadic AD are myriad, it is clear that lifestyle and overall health play a significant role. The adipocyte-derived hormone leptin has varied systemic affects, including neuropeptide release and neuroprotection. A recent study by Lieb et al. (2009) showed that individuals with low plasma leptin levels are at greater risk of developing AD, through unknown mechanisms. In this report, we show that plasma leptin is a strong negative predictor of Aβ levels in the mouse brain, supporting a protective role for the hormone in AD onset. We also show that the inhibition of Aβ accumulation is due to the downregulation of transcription of the γ-secretase components. On the other hand, β-secretase expression is either unchanged (BACE1) or increased (BACE2). Finally, we show that only presenilin 1 (PS1) is negatively correlated with plasma leptin at the protein level (p<0.0001). These data are intriguing and may highlight a role for leptin in regulating the onset of amyloid pathology and AD.
Copyright © 2013 Elsevier B.V. All rights reserved.
Figures

References
-
- Goate A, Hardy J. Twenty years of Alzheimer’s disease-causing mutations. Journal of neurochemistry. 2012;120(Suppl 1):3–8. - PubMed
-
- Querfurth HW, LaFerla FM. Alzheimer’s disease. N Engl J Med. 2010;362:329–344. - PubMed
-
- Spasic D, Annaert W. Building gamma-secretase: the bits and pieces. J Cell Sci. 2008;121:413–420. - PubMed
-
- Theuns J, Van Broeckhoven C. Transcriptional regulation of Alzheimer’s disease genes: implications for susceptibility. Hum Mol Genet. 2000;9:2383–2394. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

